CHEM02200704003 Nilamadhab Pandhy - Homi Bhabha National ...
CHEM02200704003 Nilamadhab Pandhy - Homi Bhabha National ...
CHEM02200704003 Nilamadhab Pandhy - Homi Bhabha National ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chapter 4<br />
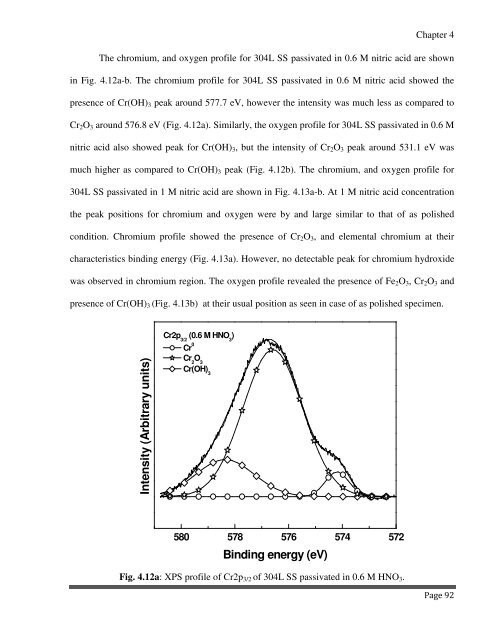
The chromium, and oxygen profile for 304L SS passivated in 0.6 M nitric acid are shown<br />
in Fig. 4.12a-b. The chromium profile for 304L SS passivated in 0.6 M nitric acid showed the<br />
presence of Cr(OH) 3 peak around 577.7 eV, however the intensity was much less as compared to<br />
Cr 2 O 3 around 576.8 eV (Fig. 4.12a). Similarly, the oxygen profile for 304L SS passivated in 0.6 M<br />
nitric acid also showed peak for Cr(OH) 3 , but the intensity of Cr 2 O 3 peak around 531.1 eV was<br />
much higher as compared to Cr(OH) 3 peak (Fig. 4.12b). The chromium, and oxygen profile for<br />
304L SS passivated in 1 M nitric acid are shown in Fig. 4.13a-b. At 1 M nitric acid concentration<br />
the peak positions for chromium and oxygen were by and large similar to that of as polished<br />
condition. Chromium profile showed the presence of Cr 2 O 3 , and elemental chromium at their<br />
characteristics binding energy (Fig. 4.13a). However, no detectable peak for chromium hydroxide<br />
was observed in chromium region. The oxygen profile revealed the presence of Fe 2 O 3 , Cr 2 O 3 and<br />
presence of Cr(OH) 3 (Fig. 4.13b) at their usual position as seen in case of as polished specimen.<br />
Intensity (Arbitrary units)<br />
Cr2p 3/2<br />
(0.6 M HNO 3<br />
)<br />
Cr 0<br />
Cr 2<br />
O 3<br />
Cr(OH) 3<br />
580 578 576 574 572<br />
Binding energy (eV)<br />
Fig. 4.12a: XPS profile of Cr2p 3/2 of 304L SS passivated in 0.6 M HNO 3 .