CHEM02200704003 Nilamadhab Pandhy - Homi Bhabha National ...
CHEM02200704003 Nilamadhab Pandhy - Homi Bhabha National ...
CHEM02200704003 Nilamadhab Pandhy - Homi Bhabha National ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 5<br />
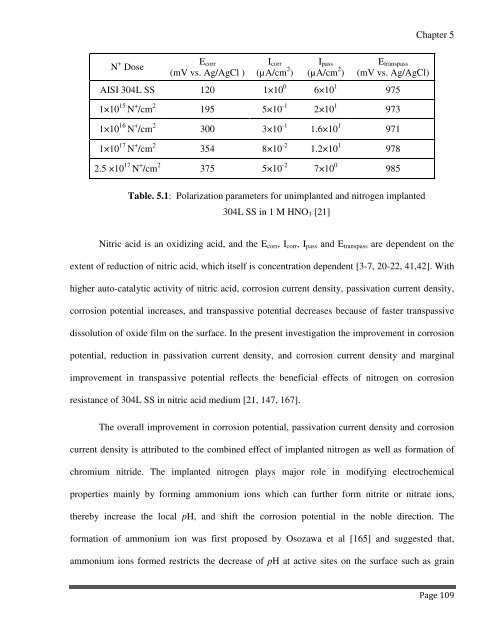
N + Dose<br />
E corr<br />
(mV vs. Ag/AgCl )<br />
I corr<br />
(µA/cm 2 )<br />
I pass<br />
(µA/cm 2 )<br />
E transpass<br />
(mV vs. Ag/AgCl)<br />
AISI 304L SS 120 1×10 0 6×10 1 975<br />
1×10 15 N + /cm 2 195 5×10 -1 2×10 1 973<br />
1×10 16 N + /cm 2 300 3×10 -1 1.6×10 1 971<br />
1×10 17 N + /cm 2 354 8×10 -2 1.2×10 1 978<br />
2.5 ×10 17 N + /cm 2 375 5×10 -2 7×10 0 985<br />
Table. 5.1: Polarization parameters for unimplanted and nitrogen implanted<br />
304L SS in 1 M HNO 3 [21]<br />
Nitric acid is an oxidizing acid, and the E corr , I corr , I pass and E transpass are dependent on the<br />
extent of reduction of nitric acid, which itself is concentration dependent [3-7, 20-22, 41,42]. With<br />
higher auto-catalytic activity of nitric acid, corrosion current density, passivation current density,<br />
corrosion potential increases, and transpassive potential decreases because of faster transpassive<br />
dissolution of oxide film on the surface. In the present investigation the improvement in corrosion<br />
potential, reduction in passivation current density, and corrosion current density and marginal<br />
improvement in transpassive potential reflects the beneficial effects of nitrogen on corrosion<br />
resistance of 304L SS in nitric acid medium [21, 147, 167].<br />
The overall improvement in corrosion potential, passivation current density and corrosion<br />
current density is attributed to the combined effect of implanted nitrogen as well as formation of<br />
chromium nitride. The implanted nitrogen plays major role in modifying electrochemical<br />
properties mainly by forming ammonium ions which can further form nitrite or nitrate ions,<br />
thereby increase the local pH, and shift the corrosion potential in the noble direction. The<br />
formation of ammonium ion was first proposed by Osozawa et al [165] and suggested that,<br />
ammonium ions formed restricts the decrease of pH at active sites on the surface such as grain