Pharmaceutical Technology: Controlled Drug Release, Volume 2
Pharmaceutical Technology: Controlled Drug Release, Volume 2
Pharmaceutical Technology: Controlled Drug Release, Volume 2
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
28 MATRIX FORMULATIONS [CH. 2<br />
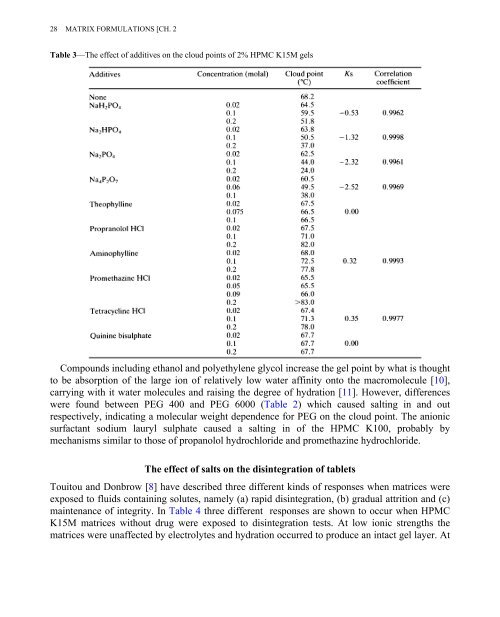
Table 3—The effect of additives on the cloud points of 2% HPMC K15M gels<br />
Compounds including ethanol and polyethylene glycol increase the gel point by what is thought<br />
to be absorption of the large ion of relatively low water affinity onto the macromolecule [10],<br />
carrying with it water molecules and raising the degree of hydration [11]. However, differences<br />
were found between PEG 400 and PEG 6000 (Table 2) which caused salting in and out<br />
respectively, indicating a molecular weight dependence for PEG on the cloud point. The anionic<br />
surfactant sodium lauryl sulphate caused a salting in of the HPMC K100, probably by<br />
mechanisms similar to those of propanolol hydrochloride and promethazine hydrochloride.<br />
The effect of salts on the disintegration of tablets<br />
Touitou and Donbrow [8] have described three different kinds of responses when matrices were<br />
exposed to fluids containing solutes, namely (a) rapid disintegration, (b) gradual attrition and (c)<br />
maintenance of integrity. In Table 4 three different responses are shown to occur when HPMC<br />
K15M matrices without drug were exposed to disintegration tests. At low ionic strengths the<br />
matrices were unaffected by electrolytes and hydration occurred to produce an intact gel layer. At