Pharmaceutical Technology: Controlled Drug Release, Volume 2
Pharmaceutical Technology: Controlled Drug Release, Volume 2
Pharmaceutical Technology: Controlled Drug Release, Volume 2
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
MATRIX FORMULATIONS [CH. 3 37<br />
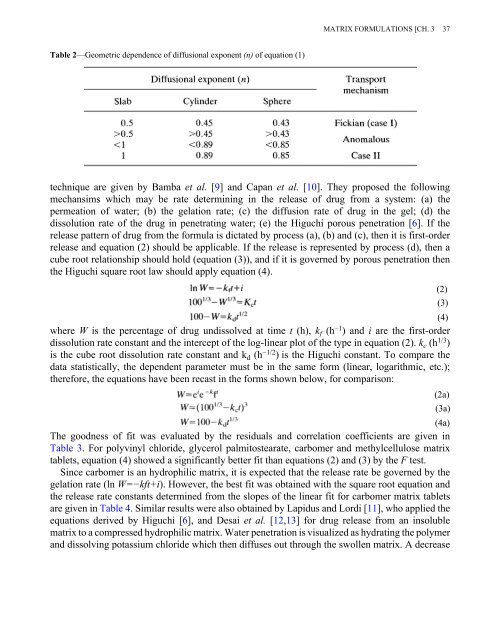
Table 2—Geometric dependence of diffusional exponent (n) of equation (1)<br />
technique are given by Bamba et al. [9] and Capan et al. [10]. They proposed the following<br />
mechansims which may be rate determining in the release of drug from a system: (a) the<br />
permeation of water; (b) the gelation rate; (c) the diffusion rate of drug in the gel; (d) the<br />
dissolution rate of the drug in penetrating water; (e) the Higuchi porous penetration [6]. If the<br />
release pattern of drug from the formula is dictated by process (a), (b) and (c), then it is first-order<br />
release and equation (2) should be applicable. If the release is represented by process (d), then a<br />
cube root relationship should hold (equation (3)), and if it is governed by porous penetration then<br />
the Higuchi square root law should apply equation (4).<br />
(2)<br />
(3)<br />
(4)<br />
where W is the percentage of drug undissolved at time t (h), k f (h −1 ) and i are the first-order<br />
dissolution rate constant and the intercept of the log-linear plot of the type in equation (2). k c (h 1/3 )<br />
is the cube root dissolution rate constant and k d (h −1/2 ) is the Higuchi constant. To compare the<br />
data statistically, the dependent parameter must be in the same form (linear, logarithmic, etc.);<br />
therefore, the equations have been recast in the forms shown below, for comparison:<br />
(2a)<br />
(3a)<br />
(4a)<br />
The goodness of fit was evaluated by the residuals and correlation coefficients are given in<br />
Table 3. For polyvinyl chloride, glycerol palmitostearate, carbomer and methylcellulose matrix<br />
tablets, equation (4) showed a significantly better fit than equations (2) and (3) by the F test.<br />
Since carbomer is an hydrophilic matrix, it is expected that the release rate be governed by the<br />
gelation rate (ln W=−kft+i). However, the best fit was obtained with the square root equation and<br />
the release rate constants determined from the slopes of the linear fit for carbomer matrix tablets<br />
are given in Table 4. Similar results were also obtained by Lapidus and Lordi [11], who applied the<br />
equations derived by Higuchi [6], and Desai et al. [12,13] for drug release from an insoluble<br />
matrix to a compressed hydrophilic matrix. Water penetration is visualized as hydrating the polymer<br />
and dissolving potassium chloride which then diffuses out through the swollen matrix. A decrease