- Page 1: ISSN-L=1454-7376 (Print)-ISSN 1454-
- Page 4 and 5: 13. CIORNEA 1 Elena, TUTU Elena, CO
- Page 6 and 7: 38. 39. 40. 41. 42. 43. 44. 45. 46.
- Page 8 and 9: 64. FILIMON V.R., NICULAUA M., MIHA
- Page 10 and 11: 91. NEGREA Roxana, ZLATI Cristina -
- Page 12 and 13: 12. BEJAN 1 Carmen, VIŞOIU Emilia
- Page 14 and 15: 38. 39. 40. 41. 42. 43. 44. 45. 46.
- Page 16 and 17: 64. 65. 66. 67. 68. 69. 70. 71. 72.
- Page 18 and 19: compoziţii vegetale pentru amenaj
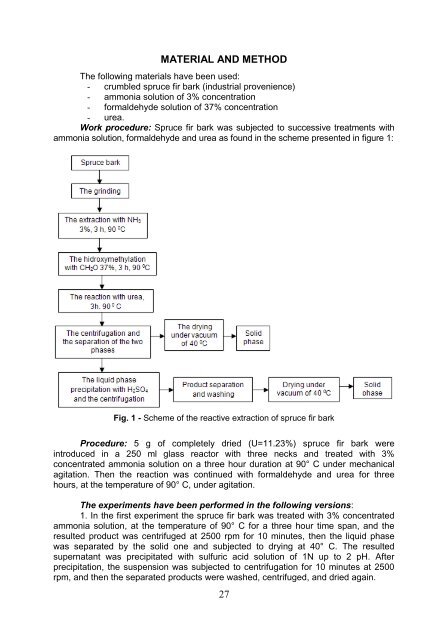
- Page 20 and 21: INTRODUCTION Due to the increased f
- Page 22 and 23: Salicylates provided an increased y
- Page 24 and 25: total mass. However, it is required
- Page 28 and 29: 2. In the second experiment the spr
- Page 30 and 31: CONCLUSIONS 1. The extraction of sp
- Page 32 and 33: INTRODUCTION Lately, one may notice
- Page 34 and 35: ions leads to a retention rate grow
- Page 36 and 37: CONCLUSIONS 1. The stability of tre
- Page 38 and 39: fundamental process in coupled dyna
- Page 40 and 41: x� 1 � x 2 x� 2 � x� 3
- Page 42 and 43: Fig. 7 - (2y1(t) -, x1(t) -, for p=
- Page 44 and 45: showed that the existence of fracta
- Page 46 and 47: Fig. 3 - The fractal dimension for
- Page 48 and 49: From figure 6 we can see that the e
- Page 50 and 51: INTRODUCTION Nichel as ultramicroel
- Page 52 and 53: Table 2 POD activity (conventional
- Page 54 and 55: REFERENCES 1. Brown P.H., Welch R.M
- Page 56 and 57: Actually, SSR markers are considere
- Page 58 and 59: Table 1 (continuation) Inherintance
- Page 60 and 61: Drofa Rf genotype was positioned in
- Page 62 and 63: Center for Biotechnology Informatio
- Page 64 and 65: Fig. 2 - RRM_1- RNA recognition mot
- Page 66 and 67: 4. Mackenzie S.A., McIntosh L., 199
- Page 68 and 69: MATERIAL AND METHOD Six fertility r
- Page 70 and 71: Fig. 1 - Densitometry analysis of O
- Page 72 and 73: CONCLUSIONS 1. Screening of three O
- Page 74 and 75: The first flow cytometers quantifie
- Page 76 and 77:
Our data confirm the previous resul
- Page 78 and 79:
7. Dydak M., Kolano B., Nowak T., S
- Page 80 and 81:
prezenţa şi tipul aberaţiilor cr
- Page 82 and 83:
The value of the mitotic index was
- Page 84 and 85:
CONCLUSIONS 1. The value of the mit
- Page 86 and 87:
INTRODUCTION Like the other plants,
- Page 88 and 89:
Fig. 3 - Histogram representing the
- Page 90 and 91:
Among the tested control plants, th
- Page 92 and 93:
their control techniques. The main
- Page 94 and 95:
figure 2, and, in their analysis it
- Page 96 and 97:
CONCLUSIONS The analysis of experim
- Page 98 and 99:
metabolism can be easily detected b
- Page 100 and 101:
PAL activity (∆E/mg prot.) PAL ac
- Page 102 and 103:
Academiei de Ştiinţe a Moldovei.
- Page 104 and 105:
efectuate investigaţii biochimice
- Page 106 and 107:
UI compared to 31,53 UI, the value
- Page 108 and 109:
3. The evolution of superoxide dism
- Page 110 and 111:
the pyridazine derivatives in growt
- Page 112 and 113:
Pyridazine derivatives tested did n
- Page 114 and 115:
8. Drochioiu G., Maftei M., Mangala
- Page 116 and 117:
according to the classification to
- Page 118 and 119:
formazan/g, a mixture of micronutri
- Page 120 and 121:
4. At 7 days after inoculation of t
- Page 122 and 123:
the root types of vascular bundles
- Page 124 and 125:
cells disposed more compacted at su
- Page 126 and 127:
CONCLUSIONS 1. The general structur
- Page 128 and 129:
INTRODUCTION Never in the history o
- Page 130 and 131:
Attracting social partners in the e
- Page 132 and 133:
CONCLUSIONS 1. Evaluator and tutors
- Page 134 and 135:
firms seek resources and new ways t
- Page 136 and 137:
companies in the wine sector. Indic
- Page 138 and 139:
From the analysis of figure 1 can b
- Page 140 and 141:
warning about the practice of copyi
- Page 142 and 143:
success to an extent far greater th
- Page 144 and 145:
CONCLUSIONS 1. At regional level th
- Page 146 and 147:
team that he leads, establishing re
- Page 148 and 149:
Nr. crt. NAME OF CROPS Areas cultiv
- Page 150 and 151:
management skills necessary to admi
- Page 152 and 153:
personalities. The education by mea
- Page 154 and 155:
The final part of the activities wa
- Page 156 and 157:
CONCLUSIONS The activities carried
- Page 158 and 159:
din punct de vedere al creşterii v
- Page 160 and 161:
percentage of + 58 %. The productio
- Page 162 and 163:
The second component reflects the l
- Page 164 and 165:
It should be noted that the net inc
- Page 166 and 167:
CONCLUSIONS 1. Studies have shown t
- Page 168 and 169:
variety were given mineral fertiliz
- Page 170 and 171:
40.7 g compared to the control. In
- Page 172 and 173:
In super intensive orchards of spec
- Page 174 and 175:
3 years of fruiting, 72.9 t/ha. The
- Page 176 and 177:
Replacing the old varieties, that h
- Page 178 and 179:
Golden Reinders varieties on the br
- Page 180 and 181:
varieties introduced in the country
- Page 182 and 183:
and H. Gilbert (1843), at the Rotha
- Page 184 and 185:
Nr. crt. Table 1 Distances and dens
- Page 186 and 187:
CONCLUSIONS 1. Technological factor
- Page 188 and 189:
Knowledge of the main agrobiologica
- Page 190 and 191:
The following graph, the diameter g
- Page 192 and 193:
CONCLUSIONS 1. The analysis main mo
- Page 194 and 195:
Vegetable production is marked by a
- Page 196 and 197:
vegetable sprouts. Optimum growing
- Page 198 and 199:
Ecological and physiological charac
- Page 200 and 201:
MATERIAL AND METHOD The carrying ou
- Page 202 and 203:
Question: “Would you buy ecologic
- Page 204 and 205:
Thus, 12.9% out of the persons inte
- Page 206 and 207:
MATERIAL AND METHOD The biological
- Page 208 and 209:
No crt. 1 Results regarding the fre
- Page 210 and 211:
CONCLUSIONS 1. The meteorological-p
- Page 212 and 213:
MATERIAL AND METHOD The research wa
- Page 214 and 215:
In the stationary microfarm Maxim o
- Page 216 and 217:
Tomato Granadero F1 row Tomato Gran
- Page 218 and 219:
MATERIAL AND METHOD The breeding wo
- Page 220 and 221:
No. Followed character The main cha
- Page 222 and 223:
L9 L10 Mt Fig. 1 - The selection of
- Page 224 and 225:
INTRODUCTION Increasing demand for
- Page 226 and 227:
REFERENCES 1. Gozob T., Micu Chiria
- Page 228 and 229:
of reduced energy consumption, have
- Page 230 and 231:
The results obtained for apples pro
- Page 232 and 233:
Pruning influence on apple producti
- Page 234 and 235:
mahaleb succeeds in the NE of Roman
- Page 236 and 237:
in the maturation stadium, have cra
- Page 238 and 239:
its acidity covers an intermediary
- Page 240 and 241:
eacts with the basic solution, the
- Page 242 and 243:
and mildew vulnerability, obtained
- Page 244 and 245:
Analyzing data on the amount of bio
- Page 246 and 247:
INTRODUCTION Numerous scientists ar
- Page 248 and 249:
- Arthemisia annua, cultivated in c
- Page 250 and 251:
much more decorative, framing very
- Page 252 and 253:
potential, were realised both on na
- Page 254 and 255:
flowers grouped in umbelliform infl
- Page 256 and 257:
3. Function of ornamental features
- Page 258 and 259:
and have species with different eco
- Page 260 and 261:
It is cultivated as ornamental gras
- Page 262 and 263:
„Silberfeder Miscanthus sinensis
- Page 264 and 265:
INTRODUCTION Located in the SE of R
- Page 266 and 267:
3. Sedum urvillei DC. (sin. Sedum s
- Page 268 and 269:
CONCLUSIONS 1. The spontaneous flor
- Page 270 and 271:
1. - the bush shape; 2. - the vigou
- Page 272 and 273:
Table 4 The quality evaluation shee
- Page 274 and 275:
value of roses. Through this we can
- Page 276 and 277:
three meters between rows and one m
- Page 278 and 279:
Table 3 Fruit production of apple c
- Page 280 and 281:
CONCLUSIONS The production of apple
- Page 282 and 283:
INTRODUCTION Researches whose resul
- Page 284 and 285:
The spatial distribution of suitabi
- Page 286 and 287:
3. The most favorable for the wine
- Page 288 and 289:
MATERIAL AND METHOD The ecological
- Page 290 and 291:
CONCLUSIONS The ecological index ca
- Page 292 and 293:
of rose wines differing according t
- Page 294 and 295:
The changes of color components are
- Page 296 and 297:
Blending means - beyond the combina
- Page 298 and 299:
The V[p,s] property values for the
- Page 300 and 301:
No major changes of the hierarchy a
- Page 302 and 303:
another essential condition: the ca
- Page 304 and 305:
having a fish tradition, for a cons
- Page 306 and 307:
The environment conditions may favo
- Page 308 and 309:
Fig. 1 - Chemical formula of vitami
- Page 310 and 311:
substances with a protective role d
- Page 312 and 313:
~ Is allergic activities (www.trata
- Page 314 and 315:
and Grădinariu G, 2000), cherry pr
- Page 316 and 317:
Fruits moisture ranged between 79.6
- Page 318 and 319:
5. The data obtained, are within th
- Page 320 and 321:
Plums contain large quantities of s
- Page 322 and 323:
Regarding the fact that all cultiva
- Page 324 and 325:
other compounds, while at the Dâmb
- Page 326 and 327:
statistic-mathematical multi-variat
- Page 328 and 329:
In order to ensure more fidelity in
- Page 330 and 331:
INTRODUCTION Climate change has an
- Page 332 and 333:
Fig. 2 - TblClima table structure T
- Page 334 and 335:
and provides information on phenolo
- Page 336 and 337:
INTRODUCTION It is a common knowled
- Page 338 and 339:
A tendency towards enhancement of l
- Page 340 and 341:
At the same time, the results of yi
- Page 342 and 343:
phénolique constitue une donne ind
- Page 344 and 345:
plus petites que pur le Merlot ou l
- Page 346 and 347:
Suite aux dégustations organolépt
- Page 348 and 349:
wines. A fourth variety, Gewürtztr
- Page 350 and 351:
The highest quantities are found in
- Page 352 and 353:
CONCLUSIONS 1. In Tămâioasă rom
- Page 354 and 355:
INTRODUCTION The physical-chemical
- Page 356 and 357:
N o Technologi cal variant Alcoho l
- Page 358 and 359:
REFERENCES 1. Cotea D.V., 1985 - Tr
- Page 360 and 361:
asic wine and the yeasts triggering
- Page 362 and 363:
Fig. 2 - Dynamics of reducing sugar
- Page 364 and 365:
Pressure was determined since the 1
- Page 366 and 367:
character that may be frequently fo
- Page 368 and 369:
To totally eliminate the germs of b
- Page 370 and 371:
Aluminum vessels used for the prepa
- Page 372 and 373:
Influence of technological phases o
- Page 374 and 375:
Table 4 Losses of vitamin C recorde
- Page 376 and 377:
ascorbic, E301 sodium ascorbate, E3
- Page 378 and 379:
anthocyanin pigments, flavonoids, t
- Page 380 and 381:
economically speaking, due to very
- Page 382 and 383:
REFERENCES 1. Adams J. B., 1973 - T
- Page 384 and 385:
with ascorbic acid are major antiox
- Page 386 and 387:
cabbages, which have antioxidant ac
- Page 388 and 389:
5. Correlation between total phenol
- Page 390 and 391:
MATERIAL AND METHOD Experiences wer
- Page 392 and 393:
compared to the control and lowest
- Page 394 and 395:
and development, increased producti
- Page 396 and 397:
Conversely, the cost increases and
- Page 398 and 399:
CONCLUSIONS 1. In the central area
- Page 400 and 401:
INTRODUCTION The physical degradati
- Page 402 and 403:
The laboratory test rig has the fol
- Page 404 and 405:
Acknowledgements. The laboratory te
- Page 406 and 407:
Factor B = NITROGEN (kg N / ha): b1
- Page 408 and 409:
Table 3 Influence of nitrogen and p
- Page 410 and 411:
4. Regarding the influence of incre
- Page 412 and 413:
the two objective and important cha
- Page 414 and 415:
- very hard summer consistency on s
- Page 416 and 417:
weather and antropic factors of the
- Page 418 and 419:
Wander and co., 2002 considers that
- Page 420 and 421:
Ecological climatic factors Average
- Page 422 and 423:
7. Januszek K, 1999 - Actywnosc enz
- Page 424 and 425:
using the most suitable rootstocks
- Page 426 and 427:
Soil porosity. Large pore diverse l
- Page 428 and 429:
low, which does not influence the d
- Page 430 and 431:
2005, 2009 a, 2009 b, Borza I. et a
- Page 432 and 433:
Table 5 The total water consumption
- Page 434 and 435:
CONCLUSIONS 1. At unirrigated potat
- Page 436 and 437:
irrigation systems the paper studie
- Page 438 and 439:
The crop coefficients (Kc) for the
- Page 440 and 441:
for respectively month. The differe
- Page 442 and 443:
MATERIAL AND METHOD In order to per
- Page 444 and 445:
In order to facilitate the access o
- Page 446 and 447:
The water sources absorbed by the b
- Page 448 and 449:
compaction of the cultivated horizo
- Page 450 and 451:
machinery wheals, we can observe a
- Page 452 and 453:
CONCLUSIONS 1. The increase of the
- Page 454 and 455:
These compounds are efficient in co
- Page 456 and 457:
CONCLUSIONS The fight against this
- Page 458 and 459:
INTRODUCTION Timis River, the riche
- Page 460 and 461:
Gavojdia sampling point is located
- Page 462 and 463:
0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 0.0
- Page 464 and 465:
Recording as changes in the structu
- Page 466 and 467:
The earthworms species collected in
- Page 468 and 469:
MATERIAL AND METHOD The research wa
- Page 470 and 471:
In the Farm Rediu Iasi from table 3
- Page 472 and 473:
36 63 472 82 Vasile Adamachi Rediu
- Page 474 and 475:
gathered from May to July. Gatherin
- Page 476 and 477:
-on first harvest on 26.05 I collec
- Page 478 and 479:
6 06.06 Folicur Actelic 7 13.06 Cla
- Page 480 and 481:
MATERIAL AND METHOD The biological
- Page 482 and 483:
The differences from the three-year
- Page 484 and 485:
The analysis of data recorded in ta
- Page 486 and 487:
MATERIAL AND METHOD The research wa
- Page 488 and 489:
Nr. Phenologic phase 1 White button
- Page 490 and 491:
It has taken this choice, because i
- Page 492 and 493:
et al., 2007) and convert the dange
- Page 494 and 495:
According to data from table 3 the
- Page 496 and 497:
MATERIAL AND METHOD The experiences
- Page 498 and 499:
From the second year of culture, pl
- Page 500 and 501:
During this study it has been found
- Page 502 and 503:
MATERIAL AND METHOD The two farms s
- Page 504 and 505:
The project contains these measures
- Page 506 and 507:
and of downstream dams that, beginn
- Page 508 and 509:
and urban creations, we link this w
- Page 510 and 511:
Also, he is one of the first creato
- Page 512 and 513:
3. In the development of such proje
- Page 514 and 515:
archeological signs with the urbani
- Page 516 and 517:
As long as the towns of medieval Mo
- Page 518 and 519:
in the palace, centrally located, o
- Page 520 and 521:
the job or to other interest points
- Page 522 and 523:
The modernization of these public m
- Page 524 and 525:
CONCLUSIONS The concept represents
- Page 526 and 527:
1975; Enache I., 2002). Research co
- Page 528 and 529:
Fig.3 - Analysis of the model As a
- Page 530 and 531:
made of plastic (for economic model
- Page 532 and 533:
The simplicity of the effect is alw
- Page 534 and 535:
a) Pyrus calleryana b) Pyrus amygda
- Page 536 and 537:
The quantity of colour plays an imp
- Page 538 and 539:
unique, colourful foliage, like the
- Page 540 and 541:
Another decorative specie is Japane
- Page 542 and 543:
foliage greens, but in other colour
- Page 544 and 545:
imagination was regarded as having
- Page 546 and 547:
specific way of interpreting realit
- Page 548 and 549:
MATERIAL AND METHOD The plant mater
- Page 550 and 551:
following varieties in the South of
- Page 552 and 553:
contents of the above compounds is
- Page 554 and 555:
a number of about 40 ample windthro
- Page 556 and 557:
Fig. 2 - EEF index values before wi
- Page 558 and 559:
ecoprotective effectiveness is main
- Page 560 and 561:
independence, too (Adams S. et al.,
- Page 562 and 563:
RESULTS AND DISCUSSIONS Results of
- Page 564 and 565:
2,3°C the specific cost is 16,07
- Page 566 and 567:
INTRODUCTION Dealu Bujorului vineya
- Page 568 and 569:
In August, when the grape vine begi
- Page 570 and 571:
So, the wines made by maceration-fe
- Page 572 and 573:
Forest "Dumbrava Sibiului" in 1900-
- Page 574 and 575:
Family P A P I L I O N I D A E Genu
- Page 576 and 577:
thousands of collection of this dat
- Page 578:
Editorial Consultant: Vasile VÎNTU