2008 Clinical Practice Guidelines - Canadian Diabetes Association
2008 Clinical Practice Guidelines - Canadian Diabetes Association
2008 Clinical Practice Guidelines - Canadian Diabetes Association
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>2008</strong> CLINICAL PRACTICE GUIDELINES<br />
S110<br />
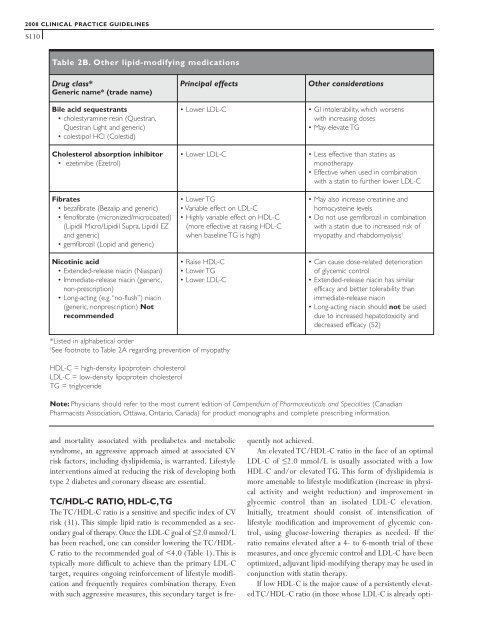
Table 2B. Other lipid-modifying medications<br />
Drug class*<br />
Generic name* (trade name)<br />
Bile acid sequestrants<br />
• cholestyramine resin (Questran,<br />
Questran Light and generic)<br />
• colestipol HCl (Colestid)<br />
Cholesterol absorption inhibitor<br />
• ezetimibe (Ezetrol)<br />
Fibrates<br />
• bezafibrate (Bezalip and generic)<br />
• fenofibrate (micronized/microcoated)<br />
(Lipidil Micro/Lipidil Supra, Lipidil EZ<br />
and generic)<br />
• gemfibrozil (Lopid and generic)<br />
Nicotinic acid<br />
• Extended-release niacin (Niaspan)<br />
• Immediate-release niacin (generic,<br />
non-prescription)<br />
• Long-acting (e.g. “no-flush”) niacin<br />
(generic, nonprescription) Not<br />
recommended<br />
and mortality associated with prediabetes and metabolic<br />
syndrome, an aggressive approach aimed at associated CV<br />
risk factors, including dyslipidemia, is warranted. Lifestyle<br />
interventions aimed at reducing the risk of developing both<br />
type 2 diabetes and coronary disease are essential.<br />
TC/HDL-C RATIO, HDL-C,TG<br />
The TC/HDL-C ratio is a sensitive and specific index of CV<br />
risk (31). This simple lipid ratio is recommended as a secondary<br />
goal of therapy. Once the LDL-C goal of ≤2.0 mmol/L<br />
has been reached, one can consider lowering the TC/HDL-<br />
C ratio to the recommended goal of