Inhibitor SourceBook™ Second Edition
Inhibitor SourceBook™ Second Edition
Inhibitor SourceBook™ Second Edition
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Apoptosis<br />
Caspase <strong>Inhibitor</strong>s<br />
Activation of caspases is one of the most widely<br />
recognized features of apoptosis. Caspases are cysteinedependent,<br />
aspartate-specific proteases. They exist as<br />
latent precursors, which, when activated by limited<br />
proteolysis, initiate the death program by destroying<br />
key components of the cellular infrastructure and<br />
activating factors that mediate damage to the cells.<br />
The mechanism of caspase activation appears to be<br />
conserved in evolution. Thus far, 14 members of the<br />
caspase family have been identified, 11 of which are<br />
present in humans. Caspases have been categorized into<br />
upstream initiators and downstream executioners. A<br />
distinctive feature of caspases is the absolute requirement<br />
of an aspartic acid residue in the substrate P 1 position.<br />
The P 4 residue is important in substrate recognition<br />
and specificity. Generally, catalysis involves a cysteine<br />
protease mechanism. The tetrapeptide corresponding to<br />
the substrate P 4 - P 1 residues is sufficient to recognize<br />
both caspase-1 and caspase-3. This also forms the basis of<br />
designing novel inhibitors of caspases.<br />
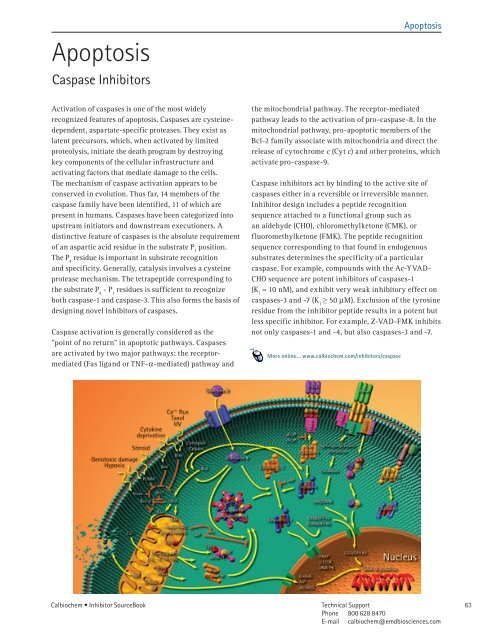
Caspase activation is generally considered as the<br />
"point of no return” in apoptotic pathways. Caspases<br />
are activated by two major pathways: the receptormediated<br />
(Fas ligand or TNF-a-mediated) pathway and<br />
Calbiochem • <strong>Inhibitor</strong> SourceBook<br />
Apoptosis<br />
the mitochondrial pathway. The receptor-mediated<br />
pathway leads to the activation of pro-caspase-8. In the<br />
mitochondrial pathway, pro-apoptotic members of the<br />
Bcl-2 family associate with mitochondria and direct the<br />
release of cytochrome c (Cyt c) and other proteins, which<br />
activate pro-caspase-9.<br />
Caspase inhibitors act by binding to the active site of<br />
caspases either in a reversible or irreversible manner.<br />
<strong>Inhibitor</strong> design includes a peptide recognition<br />
sequence attached to a functional group such as<br />
an aldehyde (CHO), chloromethylketone (CMK), or<br />
fluoromethylketone (FMK). The peptide recognition<br />
sequence corresponding to that found in endogenous<br />
substrates determines the specificity of a particular<br />
caspase. For example, compounds with the Ac-YVAD-<br />
CHO sequence are potent inhibitors of caspases-1<br />
(K i ≈ 10 nM), and exhibit very weak inhibitory effect on<br />
caspases-3 and -7 (K i ≥ 50 mM). Exclusion of the tyrosine<br />
residue from the inhibitor peptide results in a potent but<br />
less specific inhibitor. For example, Z-VAD-FMK inhibits<br />
not only caspases-1 and -4, but also caspases-3 and -7.<br />
More online... www.calbiochem.com/inhibitors/caspase<br />
Technical Support<br />
Phone 800 628 8470<br />
E-mail calbiochem@emdbiosciences.com<br />
63