university of kwazulu-natal faculty of science and agriculture school ...
university of kwazulu-natal faculty of science and agriculture school ...
university of kwazulu-natal faculty of science and agriculture school ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
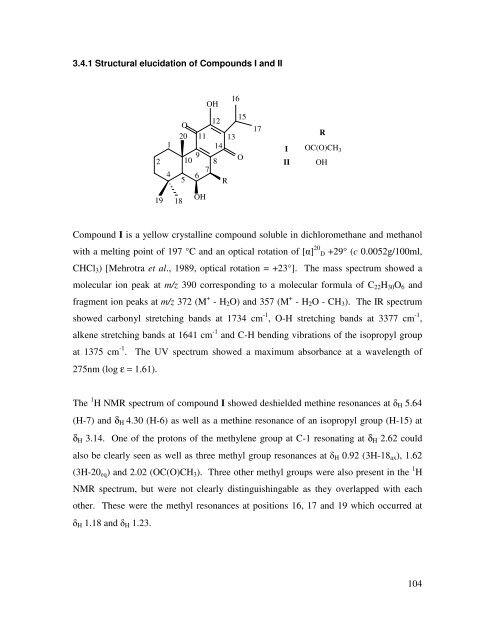
3.4.1 Structural elucidation <strong>of</strong> Compounds I <strong>and</strong> II<br />
2<br />
19<br />
1<br />
4<br />
O<br />
20<br />
18<br />
9<br />
10<br />
5<br />
11<br />
6 7<br />
OH<br />
OH<br />
12<br />
14<br />
8<br />
R<br />
13<br />
16<br />
15<br />
O<br />
17<br />
I<br />
II<br />
R<br />
OC(O)CH 3<br />
Compound I is a yellow crystalline compound soluble in dichloromethane <strong>and</strong> methanol<br />
with a melting point <strong>of</strong> 197 °C <strong>and</strong> an optical rotation <strong>of</strong> [α] 20 D +29° (c 0.0052g/100ml,<br />
CHCl3) [Mehrotra et al., 1989, optical rotation = +23°]. The mass spectrum showed a<br />
molecular ion peak at m/z 390 corresponding to a molecular formula <strong>of</strong> C22H30O6 <strong>and</strong><br />
fragment ion peaks at m/z 372 (M + - H2O) <strong>and</strong> 357 (M + - H2O - CH3). The IR spectrum<br />
showed carbonyl stretching b<strong>and</strong>s at 1734 cm -1 , O-H stretching b<strong>and</strong>s at 3377 cm -1 ,<br />
alkene stretching b<strong>and</strong>s at 1641 cm -1 <strong>and</strong> C-H bending vibrations <strong>of</strong> the isopropyl group<br />
at 1375 cm -1 . The UV spectrum showed a maximum absorbance at a wavelength <strong>of</strong><br />
275nm (log ε = 1.61).<br />
The 1 H NMR spectrum <strong>of</strong> compound I showed deshielded methine resonances at δH 5.64<br />
(H-7) <strong>and</strong> δΗ 4.30 (H-6) as well as a methine resonance <strong>of</strong> an isopropyl group (H-15) at<br />
δΗ 3.14. One <strong>of</strong> the protons <strong>of</strong> the methylene group at C-1 resonating at δΗ 2.62 could<br />
also be clearly seen as well as three methyl group resonances at δH 0.92 (3H-18ax), 1.62<br />
(3H-20eq) <strong>and</strong> 2.02 (OC(O)CH3). Three other methyl groups were also present in the 1 H<br />
NMR spectrum, but were not clearly distinguishingable as they overlapped with each<br />
other. These were the methyl resonances at positions 16, 17 <strong>and</strong> 19 which occurred at<br />
δH 1.18 <strong>and</strong> δH 1.23.<br />
OH<br />
104