university of kwazulu-natal faculty of science and agriculture school ...
university of kwazulu-natal faculty of science and agriculture school ...
university of kwazulu-natal faculty of science and agriculture school ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
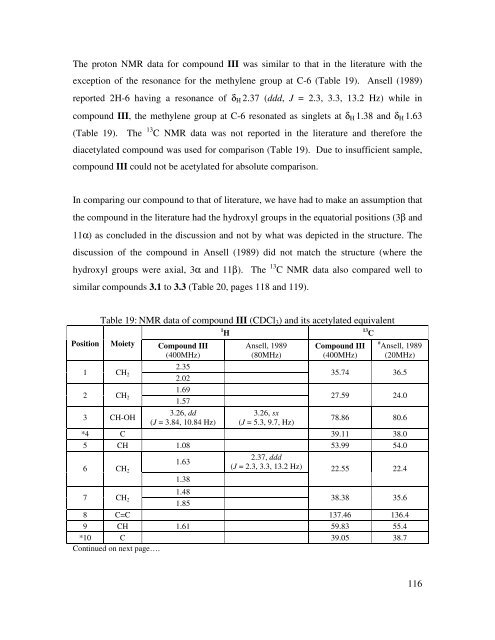
The proton NMR data for compound III was similar to that in the literature with the<br />
exception <strong>of</strong> the resonance for the methylene group at C-6 (Table 19). Ansell (1989)<br />
reported 2H-6 having a resonance <strong>of</strong> δΗ 2.37 (ddd, J = 2.3, 3.3, 13.2 Hz) while in<br />
compound III, the methylene group at C-6 resonated as singlets at δΗ 1.38 <strong>and</strong> δΗ 1.63<br />
(Table 19). The 13 C NMR data was not reported in the literature <strong>and</strong> therefore the<br />
diacetylated compound was used for comparison (Table 19). Due to insufficient sample,<br />
compound III could not be acetylated for absolute comparison.<br />
In comparing our compound to that <strong>of</strong> literature, we have had to make an assumption that<br />
the compound in the literature had the hydroxyl groups in the equatorial positions (3β <strong>and</strong><br />
11α) as concluded in the discussion <strong>and</strong> not by what was depicted in the structure. The<br />
discussion <strong>of</strong> the compound in Ansell (1989) did not match the structure (where the<br />
hydroxyl groups were axial, 3α <strong>and</strong> 11β). The 13 C NMR data also compared well to<br />
similar compounds 3.1 to 3.3 (Table 20, pages 118 <strong>and</strong> 119).<br />
Table 19: NMR data <strong>of</strong> compound III (CDCl3) <strong>and</strong> its acetylated equivalent<br />
Position Moiety<br />
1 CH2<br />
2 CH2<br />
3 CH-OH<br />
Compound III<br />
(400MHz)<br />
2.35<br />
2.02<br />
1.69<br />
1.57<br />
3.26, dd<br />
(J = 3.84, 10.84 Hz)<br />
1 H<br />
Ansell, 1989<br />
(80MHz)<br />
3.26, sx<br />
(J = 5.3, 9.7, Hz)<br />
Compound III<br />
(400MHz)<br />
13 C<br />
# Ansell, 1989<br />
(20MHz)<br />
35.74 36.5<br />
27.59 24.0<br />
78.86 80.6<br />
*4 C 39.11 38.0<br />
5 CH 1.08 53.99 54.0<br />
6 CH2<br />
1.63<br />
1.38<br />
2.37, ddd<br />
(J = 2.3, 3.3, 13.2 Hz) 22.55 22.4<br />
7 CH2<br />
1.48<br />
1.85<br />
38.38 35.6<br />
8 C=C 137.46 136.4<br />
9 CH 1.61 59.83 55.4<br />
*10 C 39.05 38.7<br />
Continued on next page….<br />
116