university of kwazulu-natal faculty of science and agriculture school ...
university of kwazulu-natal faculty of science and agriculture school ...
university of kwazulu-natal faculty of science and agriculture school ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
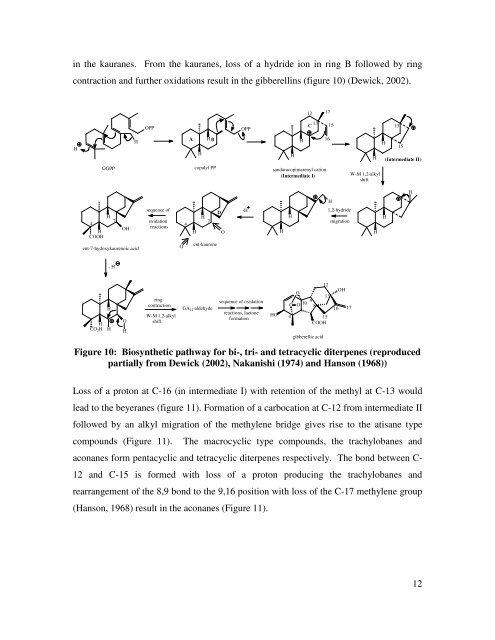
in the kauranes. From the kauranes, loss <strong>of</strong> a hydride ion in ring B followed by ring<br />
contraction <strong>and</strong> further oxidations result in the gibberellins (figure 10) (Dewick, 2002).<br />
H<br />
4<br />
H<br />
COOH<br />
GGPP<br />
H<br />
7<br />
- H<br />
H<br />
ent-7-hydoxykaurenoic acid<br />
H<br />
H<br />
CO2H H<br />
OH<br />
O<br />
H<br />
OPP<br />
sequence <strong>of</strong><br />
oxidation<br />
reactions<br />
ring<br />
contraction<br />
W-M 1,2-alkyl<br />
shift<br />
O<br />
4<br />
A H B<br />
H<br />
H<br />
copalyl PP<br />
H 7<br />
ent-kaurene<br />
GA 12-aldehyde<br />
D<br />
O<br />
OPP<br />
-H<br />
sequence <strong>of</strong> oxidation<br />
reactions, lactone<br />
formation<br />
H<br />
H<br />
13<br />
C<br />
s<strong>and</strong>aracopimarenyl cation<br />
(Intermediate I)<br />
HO<br />
H<br />
H<br />
O<br />
C O<br />
4<br />
12<br />
10 9<br />
8<br />
17<br />
16<br />
15<br />
COOH<br />
gibberellic acid<br />
12<br />
13<br />
15<br />
H<br />
1,2-hydride<br />
migration<br />
16<br />
OH<br />
17<br />
H<br />
W-M 1,2-alkyl<br />
shift<br />
H<br />
H<br />
H<br />
13<br />
15<br />
(Intermediate II)<br />
Figure 10: Biosynthetic pathway for bi-, tri- <strong>and</strong> tetracyclic diterpenes (reproduced<br />
partially from Dewick (2002), Nakanishi (1974) <strong>and</strong> Hanson (1968))<br />
Loss <strong>of</strong> a proton at C-16 (in intermediate I) with retention <strong>of</strong> the methyl at C-13 would<br />
lead to the beyeranes (figure 11). Formation <strong>of</strong> a carbocation at C-12 from intermediate II<br />
followed by an alkyl migration <strong>of</strong> the methylene bridge gives rise to the atisane type<br />
compounds (Figure 11). The macrocyclic type compounds, the trachylobanes <strong>and</strong><br />
aconanes form pentacyclic <strong>and</strong> tetracyclic diterpenes respectively. The bond between C-<br />
12 <strong>and</strong> C-15 is formed with loss <strong>of</strong> a proton producing the trachylobanes <strong>and</strong><br />
rearrangement <strong>of</strong> the 8,9 bond to the 9,16 position with loss <strong>of</strong> the C-17 methylene group<br />
(Hanson, 1968) result in the aconanes (Figure 11).<br />
H<br />
12