university of kwazulu-natal faculty of science and agriculture school ...
university of kwazulu-natal faculty of science and agriculture school ...
university of kwazulu-natal faculty of science and agriculture school ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
distinguished from the other resonances that occurred along with it. The C-9 carbon<br />
resonance showed a HMBC correlation with H-7 <strong>and</strong> CH3-20 <strong>and</strong> the C-8 carbon<br />
resonance showed a HMBC correlation to the H-7 resonance as well as the H-6<br />
resonance. These correlations confirmed the assignments <strong>of</strong> H-6 <strong>and</strong> H-7 as well as C-8<br />
<strong>and</strong> C-9.<br />
The H-5, H-6 <strong>and</strong> H-7 resonances had the same slightly broadened singlet pr<strong>of</strong>ile as that<br />
<strong>of</strong> compound I <strong>and</strong> therefore the same stereochemistry would be prevalent here as well<br />
with a 6β-ax, 7β-eq-dihydroxy form. The paramagnetic shift for the 3H-20 resonance is<br />
also observed in compound II as well as for 3H-19β-ax methyl group which is more<br />
deshielded at δH 1.62 than that <strong>of</strong> 3H-18 at δH 1.06.<br />
Compound II was isolated previously from Mehrotra et al. (1989). Rasikari (2007) <strong>and</strong><br />
Hensch et al. (1975) report the 7α-axial-hydroxy isomer. However, the coupling<br />
constants <strong>of</strong> JH-6, H-7 in both Rasikari (2007) <strong>and</strong> Hensch et al. (1975) are 2.0 Hz <strong>and</strong><br />
therefore we think that these compounds may also be the 7β-equatorial-hydroxy <strong>and</strong> not<br />
the 7α-axial-hydroxy isomer. The NMR data <strong>of</strong> all three references are thus used for<br />
comparison. The 1 H NMR data compare well, with a consistent 6-7 ppm difference for<br />
Hensch et al. (1975). The 13 C NMR data compared well with that <strong>of</strong> Rasikari (2007). No<br />
13 C NMR data is given in both Mehrotra et al. (1989) <strong>and</strong> Hensch et al. (1975).<br />
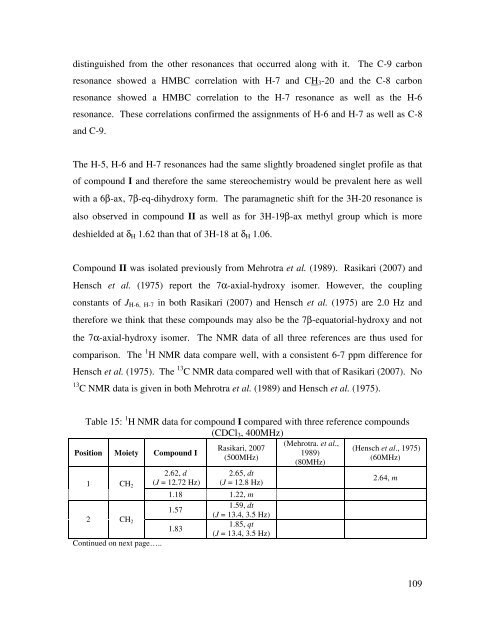
Table 15: 1 H NMR data for compound I compared with three reference compounds<br />
(CDCl3, 400MHz)<br />
Position Moiety<br />
Compound I<br />
2.62, d<br />
(J = 12.72 Hz)<br />
Rasikari, 2007<br />
(500MHz)<br />
2.65, dt<br />
(J = 12.8 Hz)<br />
1 CH2<br />
1.18 1.22, m<br />
2 CH2<br />
1.57<br />
1.83<br />
1.59, dt<br />
(J = 13.4, 3.5 Hz)<br />
1.85, qt<br />
(J = 13.4, 3.5 Hz)<br />
Continued on next page…..<br />
(Mehrotra. et al.,<br />
1989)<br />
(80MHz)<br />
(Hensch et al., 1975)<br />
(60MHz)<br />
2.64, m<br />
109