A systematic review and economic model of the effectiveness and ...
A systematic review and economic model of the effectiveness and ...
A systematic review and economic model of the effectiveness and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
98<br />
Review <strong>of</strong> <strong>economic</strong> evaluations <strong>of</strong> ADHD drug interventions in children <strong>and</strong> adolescents<br />
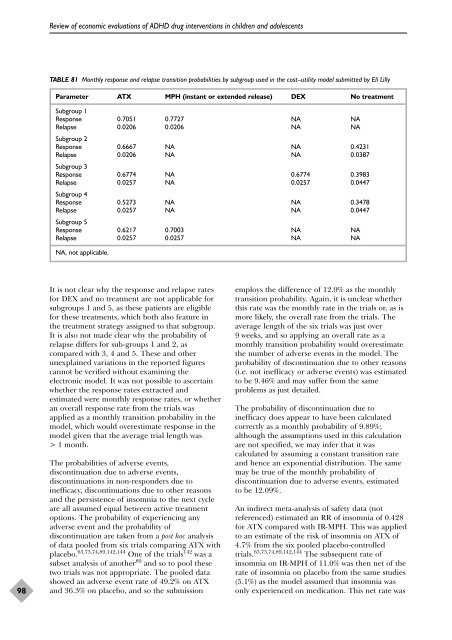
TABLE 81 Monthly response <strong>and</strong> relapse transition probabilities by subgroup used in <strong>the</strong> cost–utility <strong>model</strong> submitted by Eli Lilly<br />
Parameter ATX MPH (instant or extended release) DEX No treatment<br />
Subgroup 1<br />
Response 0.7051 0.7727 NA NA<br />
Relapse<br />
Subgroup 2<br />
0.0206 0.0206 NA NA<br />
Response 0.6667 NA NA 0.4231<br />
Relapse<br />
Subgroup 3<br />
0.0206 NA NA 0.0387<br />
Response 0.6774 NA 0.6774 0.3983<br />
Relapse<br />
Subgroup 4<br />
0.0257 NA 0.0257 0.0447<br />
Response 0.5273 NA NA 0.3478<br />
Relapse<br />
Subgroup 5<br />
0.0257 NA NA 0.0447<br />
Response 0.6217 0.7003 NA NA<br />
Relapse 0.0257 0.0257 NA NA<br />
NA, not applicable.<br />
It is not clear why <strong>the</strong> response <strong>and</strong> relapse rates<br />
for DEX <strong>and</strong> no treatment are not applicable for<br />
subgroups 1 <strong>and</strong> 5, as <strong>the</strong>se patients are eligible<br />
for <strong>the</strong>se treatments, which both also feature in<br />
<strong>the</strong> treatment strategy assigned to that subgroup.<br />
It is also not made clear why <strong>the</strong> probability <strong>of</strong><br />
relapse differs for sub-groups 1 <strong>and</strong> 2, as<br />
compared with 3, 4 <strong>and</strong> 5. These <strong>and</strong> o<strong>the</strong>r<br />
unexplained variations in <strong>the</strong> reported figures<br />
cannot be verified without examining <strong>the</strong><br />
electronic <strong>model</strong>. It was not possible to ascertain<br />
whe<strong>the</strong>r <strong>the</strong> response rates extracted <strong>and</strong><br />
estimated were monthly response rates, or whe<strong>the</strong>r<br />
an overall response rate from <strong>the</strong> trials was<br />
applied as a monthly transition probability in <strong>the</strong><br />
<strong>model</strong>, which would overestimate response in <strong>the</strong><br />
<strong>model</strong> given that <strong>the</strong> average trial length was<br />
> 1 month.<br />
The probabilities <strong>of</strong> adverse events,<br />
discontinuation due to adverse events,<br />
discontinuations in non-responders due to<br />
inefficacy, discontinuations due to o<strong>the</strong>r reasons<br />
<strong>and</strong> <strong>the</strong> persistence <strong>of</strong> insomnia to <strong>the</strong> next cycle<br />
are all assumed equal between active treatment<br />
options. The probability <strong>of</strong> experiencing any<br />
adverse event <strong>and</strong> <strong>the</strong> probability <strong>of</strong><br />
discontinuation are taken from a post hoc analysis<br />
<strong>of</strong> data pooled from six trials comparing ATX with<br />
placebo. 63,73,74,89,142,144 One <strong>of</strong> <strong>the</strong> trials 142 was a<br />
subset analysis <strong>of</strong> ano<strong>the</strong>r 89 <strong>and</strong> so to pool <strong>the</strong>se<br />
two trials was not appropriate. The pooled data<br />
showed an adverse event rate <strong>of</strong> 49.2% on ATX<br />
<strong>and</strong> 36.3% on placebo, <strong>and</strong> so <strong>the</strong> submission<br />
employs <strong>the</strong> difference <strong>of</strong> 12.9% as <strong>the</strong> monthly<br />
transition probability. Again, it is unclear whe<strong>the</strong>r<br />
this rate was <strong>the</strong> monthly rate in <strong>the</strong> trials or, as is<br />
more likely, <strong>the</strong> overall rate from <strong>the</strong> trials. The<br />
average length <strong>of</strong> <strong>the</strong> six trials was just over<br />
9 weeks, <strong>and</strong> so applying an overall rate as a<br />
monthly transition probability would overestimate<br />
<strong>the</strong> number <strong>of</strong> adverse events in <strong>the</strong> <strong>model</strong>. The<br />
probability <strong>of</strong> discontinuation due to o<strong>the</strong>r reasons<br />
(i.e. not inefficacy or adverse events) was estimated<br />
to be 9.46% <strong>and</strong> may suffer from <strong>the</strong> same<br />
problems as just detailed.<br />
The probability <strong>of</strong> discontinuation due to<br />
inefficacy does appear to have been calculated<br />
correctly as a monthly probability <strong>of</strong> 9.89%;<br />
although <strong>the</strong> assumptions used in this calculation<br />
are not specified, we may infer that it was<br />
calculated by assuming a constant transition rate<br />
<strong>and</strong> hence an exponential distribution. The same<br />
may be true <strong>of</strong> <strong>the</strong> monthly probability <strong>of</strong><br />
discontinuation due to adverse events, estimated<br />
to be 12.09%.<br />
An indirect meta-analysis <strong>of</strong> safety data (not<br />
referenced) estimated an RR <strong>of</strong> insomnia <strong>of</strong> 0.428<br />
for ATX compared with IR-MPH. This was applied<br />
to an estimate <strong>of</strong> <strong>the</strong> risk <strong>of</strong> insomnia on ATX <strong>of</strong><br />
4.7% from <strong>the</strong> six pooled placebo-controlled<br />
trials. 63,73,74,89,142,144 The subsequent rate <strong>of</strong><br />
insomnia on IR-MPH <strong>of</strong> 11.0% was <strong>the</strong>n net <strong>of</strong> <strong>the</strong><br />
rate <strong>of</strong> insomnia on placebo from <strong>the</strong> same studies<br />
(5.1%) as <strong>the</strong> <strong>model</strong> assumed that insomnia was<br />
only experienced on medication. This net rate was