A systematic review and economic model of the effectiveness and ...
A systematic review and economic model of the effectiveness and ...
A systematic review and economic model of the effectiveness and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
did report that <strong>the</strong>re were no significant<br />
differences between treatments in <strong>the</strong> incidence <strong>of</strong><br />
insomnia (o<strong>the</strong>r adverse events <strong>of</strong> interest were<br />
not examined). This study did not score very well<br />
in <strong>the</strong> quality assessment, <strong>and</strong> any results should<br />
be interpreted with caution.<br />
ER-MPH low dose (≤ 20 mg/day) plus non-drug<br />
intervention versus DEX-SR plus non-drug<br />
intervention<br />
One study evaluated low-dose (≤ 20 mg/day) ER-<br />
MPH compared with DEX-SR plus non-drug<br />
intervention (Table 59; with additional information<br />
in Appendix 12). As presented above, this study<br />
did not report any hyperactivity or QoL outcomes.<br />
The scores between treatment groups were similar<br />
when assessed using <strong>the</strong> Abbreviated CTRS (see<br />
Appendix 12).<br />
Adverse events<br />
No significant differences were detected in <strong>the</strong><br />
incidence <strong>of</strong> insomnia between DEX-SR <strong>and</strong> ER-<br />
MPH. Occurrences <strong>of</strong> headache, stomach ache<br />
<strong>and</strong> loss <strong>of</strong> appetite were not recorded by patients<br />
or parents. No weight data were reported.<br />
Health Technology Assessment 2006; Vol. 10: No. 23<br />
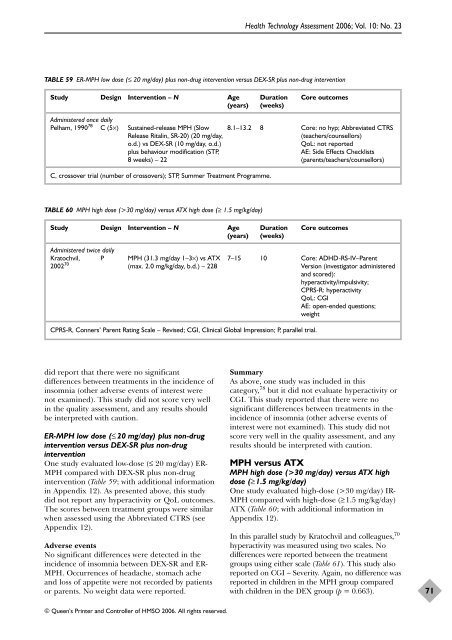
TABLE 59 ER-MPH low dose (≤ 20 mg/day) plus non-drug intervention versus DEX-SR plus non-drug intervention<br />
Study Design Intervention – N Age Duration Core outcomes<br />
(years) (weeks)<br />
Administered once daily<br />
Pelham, 1990 78 C (5×) Sustained-release MPH (Slow 8.1–13.2 8 Core: no hyp; Abbreviated CTRS<br />
Release Ritalin, SR-20) (20 mg/day, (teachers/counsellors)<br />
o.d.) vs DEX-SR (10 mg/day, o.d.) QoL: not reported<br />
plus behaviour modification (STP, AE: Side Effects Checklists<br />
8 weeks) – 22 (parents/teachers/counsellors)<br />
C, crossover trial (number <strong>of</strong> crossovers); STP, Summer Treatment Programme.<br />
TABLE 60 MPH high dose (>30 mg/day) versus ATX high dose (≥ 1.5 mg/kg/day)<br />
Study Design Intervention – N Age Duration Core outcomes<br />
(years) (weeks)<br />
Administered twice daily<br />
Kratochvil, P MPH (31.3 mg/day 1–3×) vs ATX 7–15 10 Core: ADHD-RS-IV–Parent<br />
2002 70 (max. 2.0 mg/kg/day, b.d.) – 228 Version (investigator administered<br />
<strong>and</strong> scored):<br />
hyperactivity/impulsivity;<br />
CPRS-R: hyperactivity<br />
QoL: CGI<br />
AE: open-ended questions;<br />
weight<br />
CPRS-R, Conners’ Parent Rating Scale – Revised; CGI, Clinical Global Impression; P, parallel trial.<br />
© Queen’s Printer <strong>and</strong> Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Summary<br />
As above, one study was included in this<br />
category, 78 but it did not evaluate hyperactivity or<br />
CGI. This study reported that <strong>the</strong>re were no<br />
significant differences between treatments in <strong>the</strong><br />
incidence <strong>of</strong> insomnia (o<strong>the</strong>r adverse events <strong>of</strong><br />
interest were not examined). This study did not<br />
score very well in <strong>the</strong> quality assessment, <strong>and</strong> any<br />
results should be interpreted with caution.<br />
MPH versus ATX<br />
MPH high dose (>30 mg/day) versus ATX high<br />
dose (≥1.5 mg/kg/day)<br />
One study evaluated high-dose (>30 mg/day) IR-<br />
MPH compared with high-dose (≥1.5 mg/kg/day)<br />
ATX (Table 60; with additional information in<br />
Appendix 12).<br />
In this parallel study by Kratochvil <strong>and</strong> colleagues, 70<br />
hyperactivity was measured using two scales. No<br />
differences were reported between <strong>the</strong> treatment<br />
groups using ei<strong>the</strong>r scale (Table 61). This study also<br />
reported on CGI – Severity. Again, no difference was<br />
reported in children in <strong>the</strong> MPH group compared<br />
with children in <strong>the</strong> DEX group (p = 0.663).<br />
71