A systematic review and economic model of the effectiveness and ...
A systematic review and economic model of the effectiveness and ...
A systematic review and economic model of the effectiveness and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
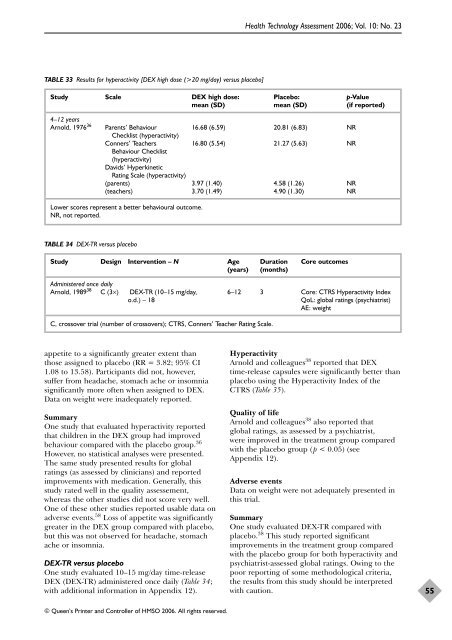
TABLE 33 Results for hyperactivity [DEX high dose (>20 mg/day) versus placebo]<br />
appetite to a significantly greater extent than<br />
those assigned to placebo (RR = 3.82; 95% CI<br />
1.08 to 13.58). Participants did not, however,<br />
suffer from headache, stomach ache or insomnia<br />
significantly more <strong>of</strong>ten when assigned to DEX.<br />
Data on weight were inadequately reported.<br />
Summary<br />
One study that evaluated hyperactivity reported<br />
that children in <strong>the</strong> DEX group had improved<br />
behaviour compared with <strong>the</strong> placebo group. 36<br />
However, no statistical analyses were presented.<br />
The same study presented results for global<br />
ratings (as assessed by clinicians) <strong>and</strong> reported<br />
improvements with medication. Generally, this<br />
study rated well in <strong>the</strong> quality assessement,<br />
whereas <strong>the</strong> o<strong>the</strong>r studies did not score very well.<br />
One <strong>of</strong> <strong>the</strong>se o<strong>the</strong>r studies reported usable data on<br />
adverse events. 58 Loss <strong>of</strong> appetite was significantly<br />
greater in <strong>the</strong> DEX group compared with placebo,<br />
but this was not observed for headache, stomach<br />
ache or insomnia.<br />
DEX-TR versus placebo<br />
One study evaluated 10–15 mg/day time-release<br />
DEX (DEX-TR) administered once daily (Table 34;<br />
with additional information in Appendix 12).<br />
Health Technology Assessment 2006; Vol. 10: No. 23<br />
Study Scale DEX high dose: Placebo: p-Value<br />
mean (SD) mean (SD) (if reported)<br />
4–12 years<br />
Arnold, 1976 36 Parents’ Behaviour 16.68 (6.59) 20.81 (6.83) NR<br />
Checklist (hyperactivity)<br />
Conners’ Teachers 16.80 (5.54) 21.27 (5.63) NR<br />
Behaviour Checklist<br />
(hyperactivity)<br />
Davids’ Hyperkinetic<br />
Rating Scale (hyperactivity)<br />
(parents) 3.97 (1.40) 4.58 (1.26) NR<br />
(teachers) 3.70 (1.49) 4.90 (1.30) NR<br />
Lower scores represent a better behavioural outcome.<br />
NR, not reported.<br />
TABLE 34 DEX-TR versus placebo<br />
Study Design Intervention – N Age Duration Core outcomes<br />
(years) (months)<br />
Administered once daily<br />
Arnold, 1989 38 C (3×) DEX-TR (10–15 mg/day, 6–12 3 Core: CTRS Hyperactivity Index<br />
o.d.) – 18 QoL: global ratings (psychiatrist)<br />
AE: weight<br />
C, crossover trial (number <strong>of</strong> crossovers); CTRS, Conners’ Teacher Rating Scale.<br />
© Queen’s Printer <strong>and</strong> Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Hyperactivity<br />
Arnold <strong>and</strong> colleagues 38 reported that DEX<br />
time-release capsules were significantly better than<br />
placebo using <strong>the</strong> Hyperactivity Index <strong>of</strong> <strong>the</strong><br />
CTRS (Table 35).<br />
Quality <strong>of</strong> life<br />
Arnold <strong>and</strong> colleagues 38 also reported that<br />
global ratings, as assessed by a psychiatrist,<br />
were improved in <strong>the</strong> treatment group compared<br />
with <strong>the</strong> placebo group (p < 0.05) (see<br />
Appendix 12).<br />
Adverse events<br />
Data on weight were not adequately presented in<br />
this trial.<br />
Summary<br />
One study evaluated DEX-TR compared with<br />
placebo. 38 This study reported significant<br />
improvements in <strong>the</strong> treatment group compared<br />
with <strong>the</strong> placebo group for both hyperactivity <strong>and</strong><br />
psychiatrist-assessed global ratings. Owing to <strong>the</strong><br />
poor reporting <strong>of</strong> some methodological criteria,<br />
<strong>the</strong> results from this study should be interpreted<br />
with caution.<br />
55