A systematic review and economic model of the effectiveness and ...
A systematic review and economic model of the effectiveness and ...
A systematic review and economic model of the effectiveness and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
14<br />
Clinical <strong>effectiveness</strong><br />
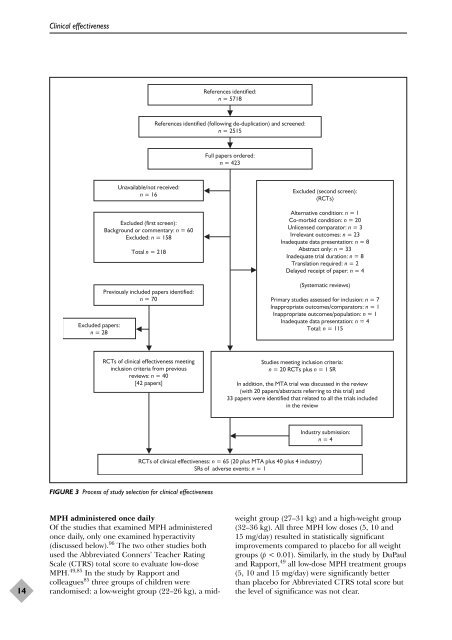
Excluded papers:<br />
n = 28<br />
Unavailable/not received:<br />
n = 16<br />
Excluded (first screen):<br />
Background or commentary: n = 60<br />
Excluded: n = 158<br />
Total n = 218<br />
Previously included papers identified:<br />
n = 70<br />
RCTs <strong>of</strong> clinical <strong>effectiveness</strong> meeting<br />
inclusion criteria from previous<br />
<strong>review</strong>s: n = 40<br />
[42 papers]<br />
FIGURE 3 Process <strong>of</strong> study selection for clinical <strong>effectiveness</strong><br />
MPH administered once daily<br />
Of <strong>the</strong> studies that examined MPH administered<br />
once daily, only one examined hyperactivity<br />
(discussed below). 96 The two o<strong>the</strong>r studies both<br />
used <strong>the</strong> Abbreviated Conners’ Teacher Rating<br />
Scale (CTRS) total score to evaluate low-dose<br />
MPH. 49,85 In <strong>the</strong> study by Rapport <strong>and</strong><br />
colleagues 85 three groups <strong>of</strong> children were<br />
r<strong>and</strong>omised: a low-weight group (22–26 kg), a mid-<br />
References identified:<br />
n = 5718<br />
References identified (following de-duplication) <strong>and</strong> screened:<br />
n = 2515<br />
Full papers ordered:<br />
n = 423<br />
Studies meeting inclusion criteria:<br />
n = 20 RCTs plus n = 1 SR<br />
In addition, <strong>the</strong> MTA trial was discussed in <strong>the</strong> <strong>review</strong><br />
(with 20 papers/abstracts referring to this trial) <strong>and</strong><br />
33 papers were identified that related to all <strong>the</strong> trials included<br />
in <strong>the</strong> <strong>review</strong><br />
RCTs <strong>of</strong> clinical <strong>effectiveness</strong>: n = 65 (20 plus MTA plus 40 plus 4 industry)<br />
SRs <strong>of</strong> adverse events: n = 1<br />
Excluded (second screen):<br />
(RCTs)<br />
Alternative condition: n = 1<br />
Co-morbid condition: n = 20<br />
Unlicensed comparator: n = 3<br />
Irrelevant outcomes: n = 23<br />
Inadequate data presentation: n = 8<br />
Abstract only: n = 33<br />
Inadequate trial duration: n = 8<br />
Translation required: n = 2<br />
Delayed receipt <strong>of</strong> paper: n = 4<br />
(Systematic <strong>review</strong>s)<br />
Primary studies assessed for inclusion: n = 7<br />
Inappropriate outcomes/comparators: n = 1<br />
Inappropriate outcomes/population: n = 1<br />
Inadequate data presentation: n = 4<br />
Total: n = 115<br />
Industry submission:<br />
n = 4<br />
weight group (27–31 kg) <strong>and</strong> a high-weight group<br />
(32–36 kg). All three MPH low doses (5, 10 <strong>and</strong><br />
15 mg/day) resulted in statistically significant<br />
improvements compared to placebo for all weight<br />
groups (p < 0.01). Similarly, in <strong>the</strong> study by DuPaul<br />
<strong>and</strong> Rapport, 49 all low-dose MPH treatment groups<br />
(5, 10 <strong>and</strong> 15 mg/day) were significantly better<br />
than placebo for Abbreviated CTRS total score but<br />
<strong>the</strong> level <strong>of</strong> significance was not clear.