A systematic review and economic model of the effectiveness and ...
A systematic review and economic model of the effectiveness and ...
A systematic review and economic model of the effectiveness and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
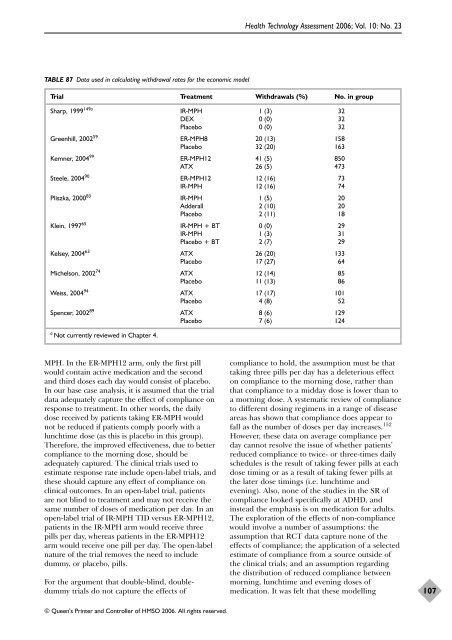
TABLE 87 Data used in calculating withdrawal rates for <strong>the</strong> <strong>economic</strong> <strong>model</strong><br />
MPH. In <strong>the</strong> ER-MPH12 arm, only <strong>the</strong> first pill<br />
would contain active medication <strong>and</strong> <strong>the</strong> second<br />
<strong>and</strong> third doses each day would consist <strong>of</strong> placebo.<br />
In our base case analysis, it is assumed that <strong>the</strong> trial<br />
data adequately capture <strong>the</strong> effect <strong>of</strong> compliance on<br />
response to treatment. In o<strong>the</strong>r words, <strong>the</strong> daily<br />
dose received by patients taking ER-MPH would<br />
not be reduced if patients comply poorly with a<br />
lunchtime dose (as this is placebo in this group).<br />
Therefore, <strong>the</strong> improved <strong>effectiveness</strong>, due to better<br />
compliance to <strong>the</strong> morning dose, should be<br />
adequately captured. The clinical trials used to<br />
estimate response rate include open-label trials, <strong>and</strong><br />
<strong>the</strong>se should capture any effect <strong>of</strong> compliance on<br />
clinical outcomes. In an open-label trial, patients<br />
are not blind to treatment <strong>and</strong> may not receive <strong>the</strong><br />
same number <strong>of</strong> doses <strong>of</strong> medication per day. In an<br />
open-label trial <strong>of</strong> IR-MPH TID versus ER-MPH12,<br />
patients in <strong>the</strong> IR-MPH arm would receive three<br />
pills per day, whereas patients in <strong>the</strong> ER-MPH12<br />
arm would receive one pill per day. The open-label<br />
nature <strong>of</strong> <strong>the</strong> trial removes <strong>the</strong> need to include<br />
dummy, or placebo, pills.<br />
For <strong>the</strong> argument that double-blind, doubledummy<br />
trials do not capture <strong>the</strong> effects <strong>of</strong><br />
© Queen’s Printer <strong>and</strong> Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 23<br />
Trial Treatment Withdrawals (%) No. in group<br />
Sharp, 1999 149a<br />
Greenhill, 2002 59<br />
Kemner, 2004 99<br />
Steele, 2004 90<br />
Pliszka, 2000 83<br />
Klein, 1997 65<br />
Kelsey, 2004 63<br />
Michelson, 2002 74<br />
Weiss, 2004 94<br />
Spencer, 2002 89<br />
a Not currently <strong>review</strong>ed in Chapter 4.<br />
IR-MPH 1 (3) 32<br />
DEX 0 (0) 32<br />
Placebo 0 (0) 32<br />
ER-MPH8 20 (13) 158<br />
Placebo 32 (20) 163<br />
ER-MPH12 41 (5) 850<br />
ATX 26 (5) 473<br />
ER-MPH12 12 (16) 73<br />
IR-MPH 12 (16) 74<br />
IR-MPH 1 (5) 20<br />
Adderall 2 (10) 20<br />
Placebo 2 (11) 18<br />
IR-MPH + BT 0 (0) 29<br />
IR-MPH 1 (3) 31<br />
Placebo + BT 2 (7) 29<br />
ATX 26 (20) 133<br />
Placebo 17 (27) 64<br />
ATX 12 (14) 85<br />
Placebo 11 (13) 86<br />
ATX 17 (17) 101<br />
Placebo 4 (8) 52<br />
ATX 8 (6) 129<br />
Placebo 7 (6) 124<br />
compliance to hold, <strong>the</strong> assumption must be that<br />
taking three pills per day has a deleterious effect<br />
on compliance to <strong>the</strong> morning dose, ra<strong>the</strong>r than<br />
that compliance to a midday dose is lower than to<br />
a morning dose. A <strong>systematic</strong> <strong>review</strong> <strong>of</strong> compliance<br />
to different dosing regimens in a range <strong>of</strong> disease<br />
areas has shown that compliance does appear to<br />
fall as <strong>the</strong> number <strong>of</strong> doses per day increases. 152<br />
However, <strong>the</strong>se data on average compliance per<br />
day cannot resolve <strong>the</strong> issue <strong>of</strong> whe<strong>the</strong>r patients’<br />
reduced compliance to twice- or three-times daily<br />
schedules is <strong>the</strong> result <strong>of</strong> taking fewer pills at each<br />
dose timing or as a result <strong>of</strong> taking fewer pills at<br />
<strong>the</strong> later dose timings (i.e. lunchtime <strong>and</strong><br />
evening). Also, none <strong>of</strong> <strong>the</strong> studies in <strong>the</strong> SR <strong>of</strong><br />
compliance looked specifically at ADHD, <strong>and</strong><br />
instead <strong>the</strong> emphasis is on medication for adults.<br />
The exploration <strong>of</strong> <strong>the</strong> effects <strong>of</strong> non-compliance<br />
would involve a number <strong>of</strong> assumptions: <strong>the</strong><br />
assumption that RCT data capture none <strong>of</strong> <strong>the</strong><br />
effects <strong>of</strong> compliance; <strong>the</strong> application <strong>of</strong> a selected<br />
estimate <strong>of</strong> compliance from a source outside <strong>of</strong><br />
<strong>the</strong> clinical trials; <strong>and</strong> an assumption regarding<br />
<strong>the</strong> distribution <strong>of</strong> reduced compliance between<br />
morning, lunchtime <strong>and</strong> evening doses <strong>of</strong><br />
medication. It was felt that <strong>the</strong>se <strong>model</strong>ling<br />
107