A systematic review and economic model of the effectiveness and ...
A systematic review and economic model of the effectiveness and ...
A systematic review and economic model of the effectiveness and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
58<br />
Clinical <strong>effectiveness</strong><br />
Summary<br />
Only one study in this category examined<br />
hyperactivity as an outcome. 62 In this study,<br />
children who received DEX in combination with<br />
academic instruction <strong>and</strong> <strong>the</strong>rapeutic recreation<br />
had better behaviour than those in <strong>the</strong> nonintervention<br />
group when assessed using <strong>the</strong> CTRS<br />
<strong>and</strong> <strong>the</strong> Children’s Psychiatric Rating Scale, but<br />
not when assessed using <strong>the</strong> CPRS. No studies in<br />
this category evaluated CGI. Owing to <strong>the</strong> poor<br />
reporting <strong>of</strong> some methodological criteria, <strong>the</strong><br />
results from this study should be interpreted with<br />
caution.<br />
DEX high dose (>20 mg/day) plus non-drug<br />
intervention versus non-drug intervention<br />
One study evaluated high-dose (>20 mg/day) DEX<br />
plus non-drug intervention compared with a nondrug<br />
intervention (Table 40; with additional<br />
information in Appendix 12). In this crossover<br />
study, drug treatment was combined with a<br />
multidisciplinary behaviour modification<br />
programme <strong>and</strong> low monoamine diet. Although<br />
<strong>the</strong> authors evaluated hyperactivity <strong>and</strong> CGI (with<br />
results favouring <strong>the</strong> combined treatment group),<br />
data were presented in graph form only <strong>and</strong> could<br />
not be reproduced.<br />
Adverse events<br />
A significantly higher incidence <strong>of</strong> reduced<br />
appetite <strong>and</strong> insomnia was detected during <strong>the</strong><br />
active drug phase <strong>of</strong> this trial (RR = 93.00; 95%<br />
CI 5.90 to 1467.03 <strong>and</strong> RR = 3.00; 95% CI 1.85<br />
to 4.87, respectively). Data on headache, stomach<br />
ache or weight were not reported.<br />
Summary<br />
No studies in this category reported reproducible<br />
data on hyperactivity or CGI. The one study in<br />
this category reported significantly greater<br />
incidence <strong>of</strong> loss <strong>of</strong> appetite <strong>and</strong> insomnia in <strong>the</strong><br />
combined treatment group.<br />
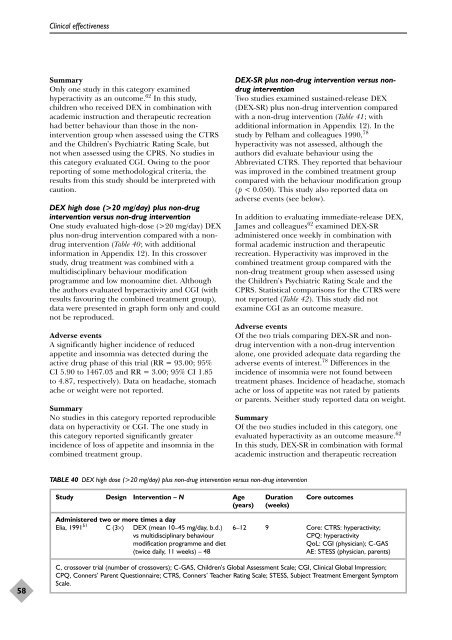
TABLE 40 DEX high dose (>20 mg/day) plus non-drug intervention versus non-drug intervention<br />
DEX-SR plus non-drug intervention versus nondrug<br />
intervention<br />
Two studies examined sustained-release DEX<br />
(DEX-SR) plus non-drug intervention compared<br />
with a non-drug intervention (Table 41; with<br />
additional information in Appendix 12). In <strong>the</strong><br />
study by Pelham <strong>and</strong> colleagues 1990, 78<br />
hyperactivity was not assessed, although <strong>the</strong><br />
authors did evaluate behaviour using <strong>the</strong><br />
Abbreviated CTRS. They reported that behaviour<br />
was improved in <strong>the</strong> combined treatment group<br />
compared with <strong>the</strong> behaviour modification group<br />
(p < 0.050). This study also reported data on<br />
adverse events (see below).<br />
In addition to evaluating immediate-release DEX,<br />
James <strong>and</strong> colleagues 62 examined DEX-SR<br />
administered once weekly in combination with<br />
formal academic instruction <strong>and</strong> <strong>the</strong>rapeutic<br />
recreation. Hyperactivity was improved in <strong>the</strong><br />
combined treatment group compared with <strong>the</strong><br />
non-drug treatment group when assessed using<br />
<strong>the</strong> Children’s Psychiatric Rating Scale <strong>and</strong> <strong>the</strong><br />
CPRS. Statistical comparisons for <strong>the</strong> CTRS were<br />
not reported (Table 42). This study did not<br />
examine CGI as an outcome measure.<br />
Adverse events<br />
Of <strong>the</strong> two trials comparing DEX-SR <strong>and</strong> nondrug<br />
intervention with a non-drug intervention<br />
alone, one provided adequate data regarding <strong>the</strong><br />
adverse events <strong>of</strong> interest. 78 Differences in <strong>the</strong><br />
incidence <strong>of</strong> insomnia were not found between<br />
treatment phases. Incidence <strong>of</strong> headache, stomach<br />
ache or loss <strong>of</strong> appetite was not rated by patients<br />
or parents. Nei<strong>the</strong>r study reported data on weight.<br />
Summary<br />
Of <strong>the</strong> two studies included in this category, one<br />
evaluated hyperactivity as an outcome measure. 62<br />
In this study, DEX-SR in combination with formal<br />
academic instruction <strong>and</strong> <strong>the</strong>rapeutic recreation<br />
Study Design Intervention – N Age Duration Core outcomes<br />
(years) (weeks)<br />
Administered two or more times a day<br />
Elia, 1991 51 C (3×) DEX (mean 10–45 mg/day, b.d.) 6–12 9 Core: CTRS: hyperactivity;<br />
vs multidisciplinary behaviour CPQ: hyperactivity<br />
modification programme <strong>and</strong> diet QoL: CGI (physician); C-GAS<br />
(twice daily, 11 weeks) – 48 AE: STESS (physician, parents)<br />
C, crossover trial (number <strong>of</strong> crossovers); C-GAS, Children’s Global Assessment Scale; CGI, Clinical Global Impression;<br />
CPQ, Conners’ Parent Questionnaire; CTRS, Conners’ Teacher Rating Scale; STESS, Subject Treatment Emergent Symptom<br />
Scale.