You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
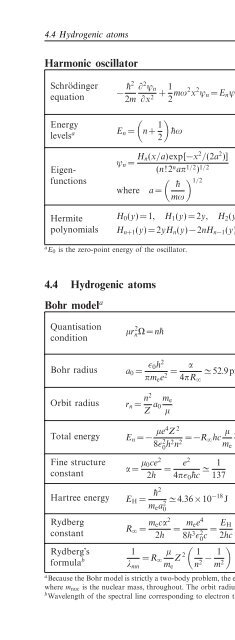
4.4 Hydrogenic atoms<br />
95<br />
Harmonic oscillator<br />
¯h (Planck constant)/(2π)<br />
Schrödinger<br />
equation<br />
− ¯h2 ∂ 2 ψ n<br />
2m ∂x 2 + 1 2 mω2 x 2 m mass<br />
ψ n = E n ψ n (4.67)<br />
ψ n nth eigenfunction<br />
x displacement<br />
(<br />
Energy<br />
levels a E n = n+ 1 )<br />
n integer ≥ 0<br />
¯hω (4.68) ω angular frequency<br />
2<br />
E n total energy in nth state<br />
ψ n = H n(x/a)exp[−x 2 /(2a 2 )]<br />
(4.69)<br />
Eigenfunctions<br />
( ) H 1/2 n Hermite polynomials<br />
(n!2 n aπ 1/2 ) 1/2<br />
¯h<br />
where a =<br />
mω<br />
Hermite<br />
polynomials<br />
H 0 (y)=1, H 1 (y)=2y, H 2 (y)=4y 2 −2<br />
H n+1 (y)=2yH n (y)−2nH n−1 (y) (4.70)<br />
y<br />
dummy variable<br />
4<br />
a E 0 is the zero-point energy <strong>of</strong> the oscillator.<br />
4.4 Hydrogenic atoms<br />
Bohr model a<br />
Quantisation<br />
condition<br />
µr 2 nΩ=n¯h (4.71)<br />
Bohr radius a 0 = ɛ 0h 2<br />
πm e e 2 = α ≃ 52.9pm (4.72)<br />
4πR ∞<br />
Orbit radius<br />
r n = n2<br />
Z a m e<br />
0<br />
µ<br />
Total energy E n = − µe4 Z 2<br />
8ɛ 2 0 h2 n 2 = −R ∞hc µ m e<br />
Z 2<br />
Fine structure<br />
constant<br />
α = µ 0ce 2<br />
2h = e2<br />
4πɛ 0¯hc ≃ 1<br />
137<br />
(4.73)<br />
n 2 (4.74)<br />
(4.75)<br />
r n nth orbit radius<br />
Ω orbital angular speed<br />
n principal quantum number<br />
(> 0)<br />
a 0 Bohr radius<br />
µ reduced mass (≃ m e )<br />
−e electronic charge<br />
Z atomic number<br />
h Planck constant<br />
¯h h/(2π)<br />
E n<br />
ɛ 0<br />
m e<br />
α<br />
total energy <strong>of</strong> nth orbit<br />
permittivity <strong>of</strong> free space<br />
electron mass<br />
fine structure constant<br />
µ 0 permeability <strong>of</strong> free space<br />
Hartree energy E H = ¯h2<br />
m e a 2 ≃ 4.36×10 −18 J (4.76) E H Hartree energy<br />
0<br />
Rydberg<br />
constant<br />
R ∞ = m ecα 2<br />
2h = m ee 4<br />
8h 3 ɛ 2 0 c = E H<br />
2hc<br />
(4.77)<br />
R ∞<br />
c<br />
Rydberg constant<br />
speed <strong>of</strong> light<br />
(<br />
Rydberg’s 1 µ 1<br />
formula b = R ∞ Z 2<br />
λ mn m e n 2 − 1 )<br />
λ mn photon wavelength<br />
m 2 (4.78)<br />
m integer >n<br />
a Because the Bohr model is strictly a two-body problem, the equations use reduced mass, µ = m e m nuc /(m e +m nuc ) ≃ m e ,<br />
where m nuc is the nuclear mass, throughout. <strong>The</strong> orbit radius is therefore the electron–nucleus distance.<br />
b Wavelength <strong>of</strong> the spectral line corresponding to electron transitions between orbits m and n.