Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
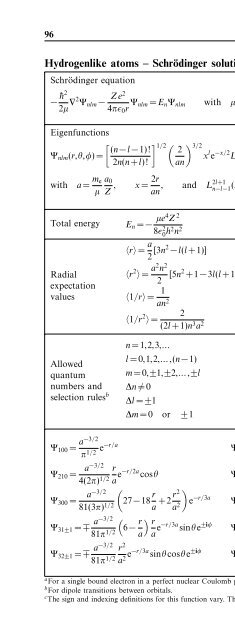
96 Quantum physics<br />
Hydrogenlike atoms – Schrödinger solution a<br />
Schrödinger equation<br />
− ¯h2<br />
2µ ∇2 Ψ nlm − Ze2<br />
4πɛ 0 r Ψ nlm = E n Ψ nlm with µ = m em nuc<br />
m e +m nuc<br />
(4.79)<br />
Eigenfunctions<br />
Ψ nlm (r,θ,φ)=<br />
with<br />
a = m e<br />
µ<br />
[ (n−l −1)!<br />
2n(n+l)!<br />
a 0<br />
Z ,<br />
] 1/2 ( ) 3/2 2<br />
x l e −x/2 L 2l+1<br />
n−l−1<br />
an<br />
(x)Y l m (θ,φ) (4.80)<br />
2r<br />
x= , and L2l+1<br />
an<br />
∑<br />
n−l−1 (x)= n−l−1<br />
k=0<br />
(l +n)!(−x) k<br />
(2l +1+k)!(n−l −1−k)!k!<br />
Total energy E n = − µe4 Z 2<br />
8ɛ 2 0 h2 n 2 (4.81)<br />
E n<br />
ɛ 0<br />
total energy<br />
permittivity <strong>of</strong> free space<br />
Radial<br />
expectation<br />
values<br />
〈r〉 = a 2 [3n2 −l(l +1)] (4.82)<br />
〈r 2 〉 = a2 n 2<br />
2 [5n2 +1−3l(l +1)] (4.83)<br />
〈1/r〉 = 1<br />
an 2 (4.84)<br />
〈1/r 2 2<br />
〉 =<br />
(2l +1)n 3 a 2 (4.85)<br />
h Planck constant<br />
m e mass<strong>of</strong>electron<br />
¯h h/2π<br />
µ reduced mass (≃ m e )<br />
m nuc mass <strong>of</strong> nucleus<br />
Ψ nlm eigenfunctions<br />
Ze charge <strong>of</strong> nucleus<br />
−e electronic charge<br />
selection rules b ∆l = ±1 (4.90)<br />
n =1,2,3,... (4.86)<br />
l =0,1,2,...,(n−1) (4.87)<br />
Allowed<br />
quantum m =0,±1,±2,...,±l (4.88)<br />
numbers and ∆n ≠ 0 (4.89)<br />
∆m =0 or ±1 (4.91)<br />
L q p associated Laguerre<br />
polynomials c<br />
a classical orbit radius, n =1<br />
r electron–nucleus separation<br />
Yl<br />
m spherical harmonics<br />
a 0 Bohr radius = ɛ 0h 2<br />
πm ee 2<br />
Ψ 100 = a−3/2<br />
π 1/2 e−r/a Ψ 200 =<br />
(2− a−3/2 r )<br />
e −r/2a<br />
4(2π) 1/2 a<br />
Ψ 210 =<br />
Ψ 300 =<br />
a−3/2 r<br />
4(2π) 1/2 a e−r/2a cosθ<br />
Ψ 21±1 = ∓ a−3/2<br />
8π 1/2 r<br />
a e−r/2a sinθe ±iφ<br />
(<br />
a−3/2<br />
27−18 r )<br />
81(3π) 1/2 a +2r2 a 2 e −r/3a Ψ 310 = 21/2 a −3/2<br />
Ψ 31±1 = ∓ a−3/2<br />
81π 1/2 (6− r a<br />
) r<br />
a e−r/3a cosθ<br />
(6− r 81π 1/2 a<br />
) r<br />
a e−r/3a sinθe ±iφ Ψ 320 = a−3/2 r 2<br />
81(6π) 1/2 a 2 e−r/3a (3cos 2 θ −1)<br />
Ψ 32±1 = ∓ a−3/2<br />
81π 1/2 r 2<br />
a 2 e−r/3a sinθcosθe ±iφ Ψ 32±2 = a−3/2<br />
162π 1/2 r 2<br />
a 2 e−r/3a sin 2 θe ±2iφ<br />
a For a single bound electron in a perfect nuclear Coulomb potential (nonrelativistic and spin-free).<br />
b For dipole transitions between orbitals.<br />
c <strong>The</strong> sign and indexing definitions for this function vary. This form is appropriate to Equation (4.80).