Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
8.8 Line radiation<br />
173<br />
8.8 Line radiation<br />
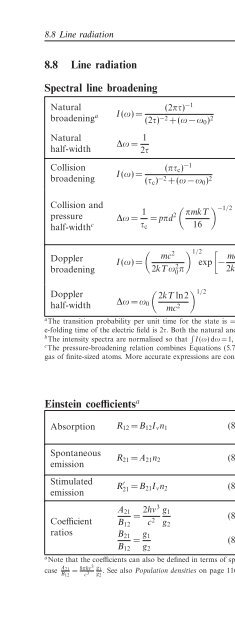
Spectral line broadening<br />
Natural<br />
broadening a<br />
Natural<br />
half-width<br />
Collision<br />
broadening<br />
Collision and<br />
pressure<br />
half-width c<br />
Doppler<br />
broadening<br />
Doppler<br />
half-width<br />
I(ω)=<br />
∆ω = 1 2τ<br />
I(ω)=<br />
(2πτ) −1<br />
(2τ) −2 +(ω −ω 0 ) 2 (8.112)<br />
(8.113)<br />
(πτ c ) −1<br />
(τ c ) −2 +(ω −ω 0 ) 2 (8.114)<br />
∆ω = 1 τ c<br />
= pπd 2 ( πmkT<br />
16<br />
( ) mc<br />
2 1/2<br />
I(ω)=<br />
2kTω0 2π exp<br />
[− mc2<br />
2kT<br />
) −1/2<br />
(8.115)<br />
(ω −ω 0 ) 2 ]<br />
ω 2 0<br />
(8.116)<br />
∆ω = ω 0<br />
( 2kT ln2<br />
mc 2 ) 1/2<br />
(8.117)<br />
I(ω) normalised intensity b<br />
τ lifetime <strong>of</strong> excited state<br />
ω angular frequency (= 2πν)<br />
∆ω<br />
ω 0<br />
τ c<br />
p<br />
d<br />
m<br />
k<br />
T<br />
c<br />
half-width at half-power<br />
centre frequency<br />
mean time between<br />
collisions<br />
pressure<br />
effective atomic diameter<br />
gas particle mass<br />
Boltzmann constant<br />
temperature<br />
speed <strong>of</strong> light<br />
ω 0<br />
a <strong>The</strong> transition probability per unit time for the state is = 1/τ. In the classical limit <strong>of</strong> a damped oscillator, the<br />
e-folding time <strong>of</strong> the electric field is 2τ. Both the natural and collision pr<strong>of</strong>iles described here are Lorentzian.<br />
b <strong>The</strong> intensity spectra are normalised so that ∫ I(ω)dω = 1, assuming ∆ω/ω 0 ≪ 1.<br />
c <strong>The</strong> pressure-broadening relation combines Equations (5.78), (5.86) and (5.89) and assumes an otherwise perfect<br />
gas <strong>of</strong> finite-sized atoms. More accurate expressions are considerably more complicated.<br />
I(ω)<br />
∆ω<br />
Einstein coefficients a<br />
Absorption R 12 = B 12 I ν n 1 (8.118)<br />
Spontaneous<br />
emission<br />
Stimulated<br />
emission<br />
R 21 = A 21 n 2 (8.119)<br />
R ′ 21 = B 21 I ν n 2 (8.120)<br />
R ij transition rate, level i → j (m −3 s −1 )<br />
B ij Einstein B coefficients<br />
I ν specific intensity <strong>of</strong> radiation field<br />
A 21 Einstein A coefficient<br />
n i number density <strong>of</strong> atoms in quantum<br />
level i (m −3 )<br />
8<br />
A 21<br />
= 2hν3 g 1<br />
h Planck constant<br />
Coefficient B 12 c 2 (8.121)<br />
g 2 ν frequency<br />
ratios B 21<br />
= g 1<br />
c speed <strong>of</strong> light<br />
(8.122)<br />
B 12 g g 2 i degeneracy <strong>of</strong> ith level<br />
a Note that the coefficients can also be defined in terms <strong>of</strong> spectral energy density, u ν =4πI ν /c rather than I ν .Inthis<br />
case A 21<br />
B = 8πhν3 g 1<br />
12 c 3 g .SeealsoPopulation densities on page 116.<br />
2