Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
100 Quantum physics<br />
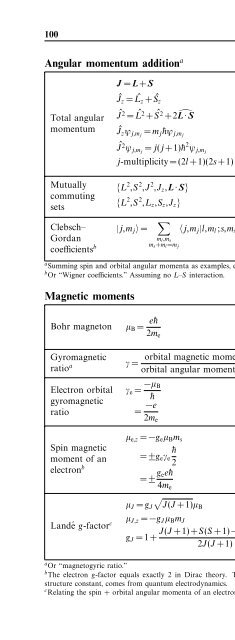
Angular momentum addition a<br />
Total angular<br />
momentum<br />
Mutually<br />
commuting<br />
sets<br />
J = L+S (4.128)<br />
Jˆ<br />
z = Lˆ<br />
z + Sˆ<br />
z (4.129)<br />
Jˆ<br />
2 = L ˆ2<br />
+ S ˆ2<br />
+2̂L·S (4.130)<br />
Jˆ<br />
z ψ j,mj = m j¯hψ j,mj (4.131)<br />
Jˆ<br />
2 ψ j,mj = j(j +1)¯h 2 ψ j,mj (4.132)<br />
j-multiplicity = (2l +1)(2s+1) (4.133)<br />
{L 2 ,S 2 ,J 2 ,J z ,L·S} (4.134)<br />
{L 2 ,S 2 ,L z ,S z ,J z } (4.135)<br />
J ,J total angular momentum<br />
L,L orbital angular<br />
momentum<br />
S,S spin angular momentum<br />
ψ eigenfunctions<br />
m j magnetic quantum<br />
number |m j |≤j<br />
j (l +s) ≥ j ≥|l −s|<br />
{} set <strong>of</strong> mutually<br />
commuting observables<br />
Clebsch– |j,m j 〉 = ∑ 〈j,m j |l,m l ;s,m s 〉|l,m l 〉|s,m s 〉<br />
Gordan<br />
m l ,m s<br />
coefficients b m s +m l =m j<br />
(4.136)<br />
a Summing spin and orbital angular momenta as examples, eigenstates |s,m s 〉 and |l,m l 〉.<br />
b Or “Wigner coefficients.” Assuming no L–S interaction.<br />
Magnetic moments<br />
Bohr magneton<br />
Gyromagnetic<br />
ratio a<br />
µ B = e¯h<br />
2m e<br />
(4.137)<br />
γ =<br />
orbital magnetic moment<br />
orbital angular momentum<br />
|·〉 eigenstates<br />
〈·|·〉 Clebsch–Gordan<br />
coefficients<br />
µ B Bohr magneton<br />
−e electronic charge<br />
¯h (Planck constant)/(2π)<br />
m e electron mass<br />
(4.138) γ gyromagnetic ratio<br />
Electron orbital<br />
gyromagnetic<br />
ratio<br />
γ e = −µ B<br />
¯h<br />
(4.139)<br />
= −e<br />
2m e<br />
(4.140)<br />
γ e<br />
electron gyromagnetic ratio<br />
µ e,z = −g e µ B m s (4.141)<br />
Spin magnetic<br />
¯h<br />
moment <strong>of</strong> an = ±g e γ e (4.142)<br />
2<br />
electron b = ± g ee¯h<br />
(4.143)<br />
4m e<br />
µ e,z z component <strong>of</strong> spin<br />
magnetic moment<br />
g e electron g-factor (≃ 2.002)<br />
m s spin quantum number (±1/2)<br />
√ µ<br />
µ J = g J J(J +1)µB (4.144) J total magnetic moment<br />
µ J,z z component <strong>of</strong> µ J<br />
µ<br />
Landé g-factor c J,z = −g J µ B m J (4.145) m J magnetic quantum number<br />
J(J +1)+S(S +1)−L(L+1) J,L,S total, orbital, and spin<br />
g J =1+<br />
2J(J +1)<br />
quantum numbers<br />
(4.146) g J Landé g-factor<br />
a Or “magnetogyric ratio.”<br />
b <strong>The</strong> electron g-factor equals exactly 2 in Dirac theory. <strong>The</strong> modification g e =2+α/π + ..., whereα is the fine<br />
structure constant, comes from quantum electrodynamics.<br />
c Relating the spin + orbital angular momenta <strong>of</strong> an electron to its total magnetic moment, assuming g e =2.