Dirac Fermions in Graphene and Graphiteâa view from angle ...
Dirac Fermions in Graphene and Graphiteâa view from angle ...
Dirac Fermions in Graphene and Graphiteâa view from angle ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
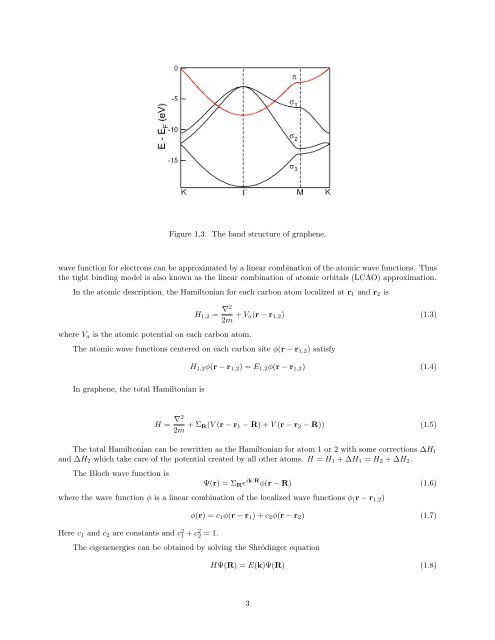
Figure 1.3. The b<strong>and</strong> structure of graphene.<br />
wave function for electrons can be approximated by a l<strong>in</strong>ear comb<strong>in</strong>ation of the atomic wave functions. Thus<br />
the tight b<strong>in</strong>d<strong>in</strong>g model is also known as the l<strong>in</strong>ear comb<strong>in</strong>ation of atomic orbitals (LCAO) approximation.<br />
In the atomic description, the Hamiltonian for each carbon atom localized at r 1 <strong>and</strong> r 2 is<br />
where V a is the atomic potential on each carbon atom.<br />
H 1,2 = ∇2<br />
2m + V a(r − r 1,2 ) (1.3)<br />
The atomic wave functions centered on each carbon site φ(r − r 1,2 ) satisfy<br />
H 1,2 φ(r − r 1,2 ) = E 1,2 φ(r − r 1,2 ) (1.4)<br />
In graphene, the total Hamiltonian is<br />
H = ∇2<br />
2m + Σ R(V (r − r 1 − R) + V (r − r 2 − R)) (1.5)<br />
The total Hamiltonian can be rewritten as the Hamiltonian for atom 1 or 2 with some corrections ∆H 1<br />
<strong>and</strong> ∆H 2 which take care of the potential created by all other atoms. H = H 1 + ∆H 1 = H 2 + ∆H 2 .<br />
The Bloch wave function is<br />
Ψ(r) = Σ R e ik·R φ(r − R) (1.6)<br />
where the wave function φ is a l<strong>in</strong>ear comb<strong>in</strong>ation of the localized wave functions φ ( r − r 1,2 )<br />
Here c 1 <strong>and</strong> c 2 are constants <strong>and</strong> c 2 1 + c 2 2 = 1.<br />
φ(r) = c 1 φ(r − r 1 ) + c 2 φ(r − r 2 ) (1.7)<br />
The eigenenergies can be obta<strong>in</strong>ed by solv<strong>in</strong>g the Shröd<strong>in</strong>ger equation<br />
HΨ(R) = E(k)Ψ(R) (1.8)<br />
3