Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
12<br />
<str<strong>on</strong>g>Guidance</str<strong>on</strong>g> <strong>on</strong> registrati<strong>on</strong><br />
Versi<strong>on</strong> 2.0 May 2012<br />
IDENTIFY IF YOU HAVE ANY ROLE<br />
WITHIN REACH:<br />
Are you manufacturer, importer, <strong>on</strong>ly<br />
representative, downstream user?<br />
secti<strong>on</strong> 2.1<br />
MAKE SURE THAT YOU KNOW WHAT<br />
YOU MANUFACTURE/IMPORT<br />
(substance/mixture/article)<br />
secti<strong>on</strong> 1.3<br />
IDENTIFY IF YOU ARE WITHIN THE<br />
SCOPE OF REACH<br />
secti<strong>on</strong> 2.2.2<br />
IDENTIFY IF YOUR SUBSTANCE IS<br />
WITHIN THE SCOPE OF<br />
REGISTRATION<br />
secti<strong>on</strong> 2.2.3<br />
CHECK WHETHER YOUR SUBSTANCE CAN BE CONSIDERED<br />
REGISTERED<br />
- have you notified it according to Directive 67/548/EEC?<br />
- does it meet the criteria menti<strong>on</strong>ed in Article 15?<br />
(Substances in plant protecti<strong>on</strong> and biocidal products)<br />
secti<strong>on</strong> 2.2.4<br />
DETERMINE IF YOU MANUFACTURE<br />
OR IMPORT THE SUBSTANCE IN<br />
QUANTITIES OF 1 TONNE OR MORE<br />
secti<strong>on</strong> 2.2.6<br />
CALCULATE THE VOLUME OF YOUR<br />
SUBSTANCE TO BE REGISTERED<br />
secti<strong>on</strong> 2.2.6<br />
DETERMINE IF YOUR SUBSTANCE IS<br />
USED AS AN ISOLATED<br />
INTERMEDIATE<br />
secti<strong>on</strong> 2.2.5<br />
DETERMINE IF YOUR SUBSTANCE IS A<br />
PHASE-IN SUBSTANCE<br />
secti<strong>on</strong> 2.3.1<br />
secti<strong>on</strong> 2.3.1.2<br />
NON PHASE-IN<br />
SUBSTANCE<br />
PHASE-IN<br />
SUBSTANCE<br />
secti<strong>on</strong> 2.3.1.1<br />
secti<strong>on</strong> 4.4<br />
PERFORM INQUIRY<br />
AND SHARE DATA<br />
BEFORE<br />
REGISTRATION<br />
no<br />
PRE-REGISTER<br />
YOUR SUBSTANCE<br />
(since 1 December 2008, <strong>on</strong>ly late<br />
pre-registrati<strong>on</strong> if c<strong>on</strong>diti<strong>on</strong>s apply)<br />
secti<strong>on</strong> 4.2<br />
yes<br />
TAKE PART IN SUBSTANCE<br />
INFORMATION EXCHANGE FORUM<br />
(SIEF) AND SHARE DATA<br />
secti<strong>on</strong> 4.3<br />
IF YOUR SUBSTANCE IS:<br />
- M/I ≥1000t or<br />
- CMR cat 1 or 2 and ≥ 1t or<br />
- R50/53 and ≥100t<br />
The registrati<strong>on</strong> period expired<br />
<strong>on</strong> 30 November 2010<br />
IF YOUR SUBSTANCE IS:<br />
- M/I ≥100t<br />
IF YOUR SUBSTANCE IS:<br />
- M/I ≥1t<br />
If you pre-registered, you will<br />
have to register your<br />
substance before<br />
1 June 2013<br />
If you pre-registered, you will<br />
have to register your<br />
substance before<br />
1 June 2018<br />
secti<strong>on</strong> 2.3.2<br />
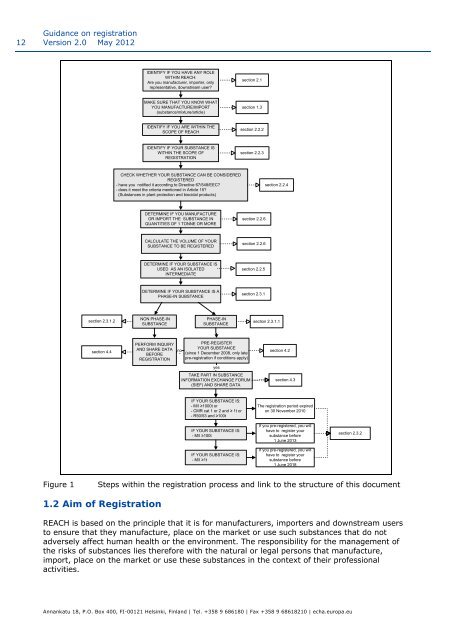
Figure 1<br />
Steps within the registrati<strong>on</strong> process and link to the structure of this document<br />
1.2 Aim of Registrati<strong>on</strong><br />
REACH is based <strong>on</strong> the principle that it is for manufacturers, importers and downstream users<br />
to ensure that they manufacture, place <strong>on</strong> the market or use such substances that do not<br />
adversely affect human health or the envir<strong>on</strong>ment. The resp<strong>on</strong>sibility for the management of<br />
the risks of substances lies therefore with the natural or legal pers<strong>on</strong>s that manufacture,<br />
import, place <strong>on</strong> the market or use these substances in the c<strong>on</strong>text of their professi<strong>on</strong>al<br />
activities.<br />
Annankatu 18, P.O. Box 400, FI-00121 Helsinki, Finland | Tel. +358 9 686180 | Fax +358 9 68618210 | echa.europa.eu