Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
52<br />
<str<strong>on</strong>g>Guidance</str<strong>on</strong>g> <strong>on</strong> registrati<strong>on</strong><br />
Versi<strong>on</strong> 2.0 May 2012<br />
3 The registrati<strong>on</strong> process<br />
Aim:<br />
The aim of this chapter is to present the informati<strong>on</strong> that the registrant has to<br />
submit as part of his registrati<strong>on</strong> and to explain how to submit it to <strong>ECHA</strong>. It also<br />
describes what a joint submissi<strong>on</strong> of registrati<strong>on</strong> data is and how to submit<br />
jointly the registrati<strong>on</strong> informati<strong>on</strong> to <strong>ECHA</strong>.<br />
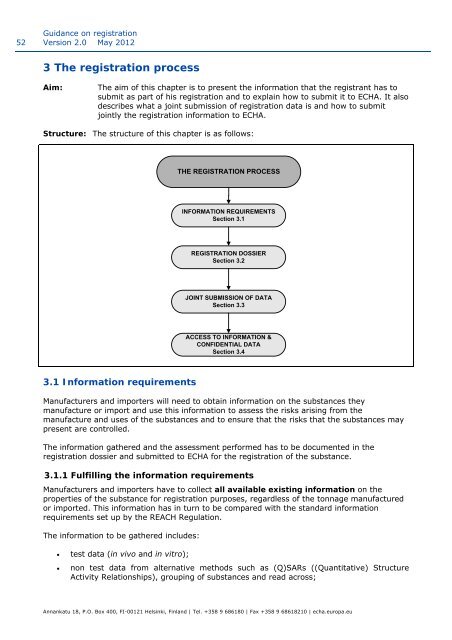
Structure: The structure of this chapter is as follows:<br />
THE REGISTRATION PROCESS<br />
INFORMATION REQUIREMENTS<br />
Secti<strong>on</strong> 3.1<br />
REGISTRATION DOSSIER<br />
Secti<strong>on</strong> 3.2<br />
JOINT SUBMISSION OF DATA<br />
Secti<strong>on</strong> 3.3<br />
ACCESS TO INFORMATION &<br />
CONFIDENTIAL DATA<br />
Secti<strong>on</strong> 3.4<br />
3.1 Informati<strong>on</strong> requirements<br />
Manufacturers and importers will need to obtain informati<strong>on</strong> <strong>on</strong> the substances they<br />
manufacture or import and use this informati<strong>on</strong> to assess the risks arising from the<br />
manufacture and uses of the substances and to ensure that the risks that the substances may<br />
present are c<strong>on</strong>trolled.<br />
The informati<strong>on</strong> gathered and the assessment performed has to be documented in the<br />
registrati<strong>on</strong> dossier and submitted to <strong>ECHA</strong> for the registrati<strong>on</strong> of the substance.<br />
3.1.1 Fulfilling the informati<strong>on</strong> requirements<br />
Manufacturers and importers have to collect all available existing informati<strong>on</strong> <strong>on</strong> the<br />
properties of the substance for registrati<strong>on</strong> purposes, regardless of the t<strong>on</strong>nage manufactured<br />
or imported. This informati<strong>on</strong> has in turn to be compared with the standard informati<strong>on</strong><br />
requirements set up by the REACH Regulati<strong>on</strong>.<br />
The informati<strong>on</strong> to be gathered includes:<br />
<br />
<br />
test data (in vivo and in vitro);<br />
n<strong>on</strong> test data from alternative methods such as (Q)SARs ((Quantitative) Structure<br />
Activity Relati<strong>on</strong>ships), grouping of substances and read across;<br />
Annankatu 18, P.O. Box 400, FI-00121 Helsinki, Finland | Tel. +358 9 686180 | Fax +358 9 68618210 | echa.europa.eu