Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<str<strong>on</strong>g>Guidance</str<strong>on</strong>g> <strong>on</strong> registrati<strong>on</strong><br />
Versi<strong>on</strong> 2.0 May 2012 121<br />
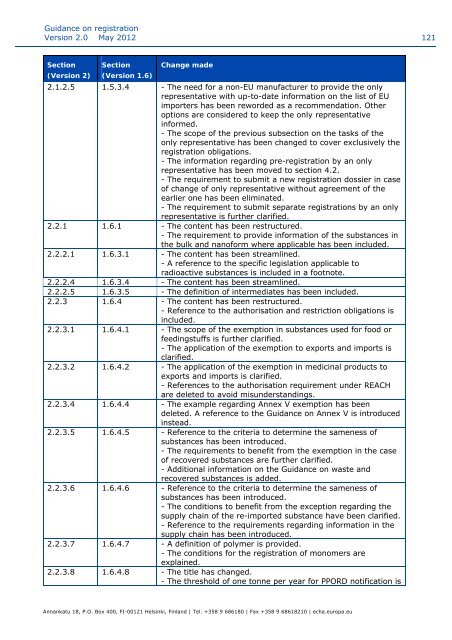
Secti<strong>on</strong> Secti<strong>on</strong> Change made<br />
(Versi<strong>on</strong> 2) (Versi<strong>on</strong> 1.6)<br />
2.1.2.5 1.5.3.4 - The need for a n<strong>on</strong>-EU manufacturer to provide the <strong>on</strong>ly<br />
representative with up-to-date informati<strong>on</strong> <strong>on</strong> the list of EU<br />
importers has been reworded as a recommendati<strong>on</strong>. Other<br />
opti<strong>on</strong>s are c<strong>on</strong>sidered to keep the <strong>on</strong>ly representative<br />
informed.<br />
- The scope of the previous subsecti<strong>on</strong> <strong>on</strong> the tasks of the<br />
<strong>on</strong>ly representative has been changed to cover exclusively the<br />
registrati<strong>on</strong> obligati<strong>on</strong>s.<br />
- The informati<strong>on</strong> regarding pre-registrati<strong>on</strong> by an <strong>on</strong>ly<br />
representative has been moved to secti<strong>on</strong> 4.2.<br />
- The requirement to submit a new registrati<strong>on</strong> dossier in case<br />
of change of <strong>on</strong>ly representative without agreement of the<br />
earlier <strong>on</strong>e has been eliminated.<br />
- The requirement to submit separate registrati<strong>on</strong>s by an <strong>on</strong>ly<br />
representative is further clarified.<br />
2.2.1 1.6.1 - The c<strong>on</strong>tent has been restructured.<br />
- The requirement to provide informati<strong>on</strong> of the substances in<br />
the bulk and nanoform where applicable has been included.<br />
2.2.2.1 1.6.3.1 - The c<strong>on</strong>tent has been streamlined.<br />
- A reference to the specific legislati<strong>on</strong> applicable to<br />
radioactive substances is included in a footnote.<br />
2.2.2.4 1.6.3.4 - The c<strong>on</strong>tent has been streamlined.<br />
2.2.2.5 1.6.3.5 - The definiti<strong>on</strong> of intermediates has been included.<br />
2.2.3 1.6.4 - The c<strong>on</strong>tent has been restructured.<br />
- Reference to the authorisati<strong>on</strong> and restricti<strong>on</strong> obligati<strong>on</strong>s is<br />
included.<br />
2.2.3.1 1.6.4.1 - The scope of the exempti<strong>on</strong> in substances used for food or<br />
feedingstuffs is further clarified.<br />
- The applicati<strong>on</strong> of the exempti<strong>on</strong> to exports and imports is<br />
clarified.<br />
2.2.3.2 1.6.4.2 - The applicati<strong>on</strong> of the exempti<strong>on</strong> in medicinal products to<br />
exports and imports is clarified.<br />
- References to the authorisati<strong>on</strong> requirement under REACH<br />
are deleted to avoid misunderstandings.<br />
2.2.3.4 1.6.4.4 - The example regarding Annex V exempti<strong>on</strong> has been<br />
deleted. A reference to the <str<strong>on</strong>g>Guidance</str<strong>on</strong>g> <strong>on</strong> Annex V is introduced<br />
instead.<br />
2.2.3.5 1.6.4.5 - Reference to the criteria to determine the sameness of<br />
substances has been introduced.<br />
- The requirements to benefit from the exempti<strong>on</strong> in the case<br />
of recovered substances are further clarified.<br />
- Additi<strong>on</strong>al informati<strong>on</strong> <strong>on</strong> the <str<strong>on</strong>g>Guidance</str<strong>on</strong>g> <strong>on</strong> waste and<br />
recovered substances is added.<br />
2.2.3.6 1.6.4.6 - Reference to the criteria to determine the sameness of<br />
substances has been introduced.<br />
- The c<strong>on</strong>diti<strong>on</strong>s to benefit from the excepti<strong>on</strong> regarding the<br />
supply chain of the re-imported substance have been clarified.<br />
- Reference to the requirements regarding informati<strong>on</strong> in the<br />
supply chain has been introduced.<br />
2.2.3.7 1.6.4.7 - A definiti<strong>on</strong> of polymer is provided.<br />
- The c<strong>on</strong>diti<strong>on</strong>s for the registrati<strong>on</strong> of m<strong>on</strong>omers are<br />
explained.<br />
2.2.3.8 1.6.4.8 - The title has changed.<br />
- The threshold of <strong>on</strong>e t<strong>on</strong>ne per year for PPORD notificati<strong>on</strong> is<br />
Annankatu 18, P.O. Box 400, FI-00121 Helsinki, Finland | Tel. +358 9 686180 | Fax +358 9 68618210 | echa.europa.eu