Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
26<br />
<str<strong>on</strong>g>Guidance</str<strong>on</strong>g> <strong>on</strong> registrati<strong>on</strong><br />
Versi<strong>on</strong> 2.0 May 2012<br />
2.2 What to register?<br />
Aim:<br />
This chapter provides an outline of which substances are subject to registrati<strong>on</strong><br />
requirements and a detailed explanati<strong>on</strong> of the circumstances under which the<br />
various exempti<strong>on</strong>s from registrati<strong>on</strong> are applicable. Because the t<strong>on</strong>nage of<br />
manufacture or import of each substance is critical in determining whether and<br />
how to register, this chapter also outlines methods for calculating the volume to<br />
be registered.<br />
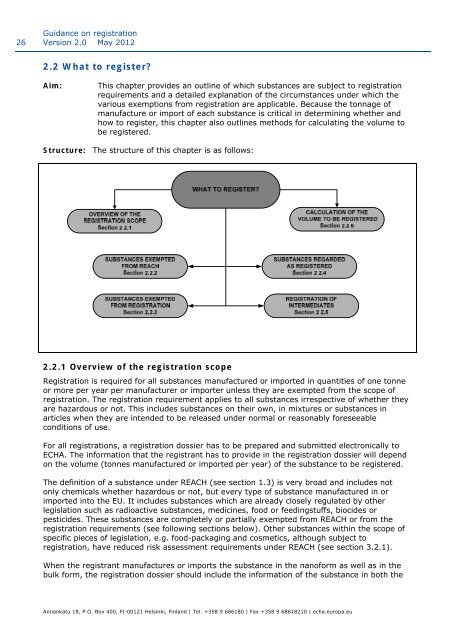
Structure: The structure of this chapter is as follows:<br />
2.2.1 Overview of the registrati<strong>on</strong> scope<br />
Registrati<strong>on</strong> is required for all substances manufactured or imported in quantities of <strong>on</strong>e t<strong>on</strong>ne<br />
or more per year per manufacturer or importer unless they are exempted from the scope of<br />
registrati<strong>on</strong>. The registrati<strong>on</strong> requirement applies to all substances irrespective of whether they<br />
are hazardous or not. This includes substances <strong>on</strong> their own, in mixtures or substances in<br />
articles when they are intended to be released under normal or reas<strong>on</strong>ably foreseeable<br />
c<strong>on</strong>diti<strong>on</strong>s of use.<br />
For all registrati<strong>on</strong>s, a registrati<strong>on</strong> dossier has to be prepared and submitted electr<strong>on</strong>ically to<br />
<strong>ECHA</strong>. The informati<strong>on</strong> that the registrant has to provide in the registrati<strong>on</strong> dossier will depend<br />
<strong>on</strong> the volume (t<strong>on</strong>nes manufactured or imported per year) of the substance to be registered.<br />
The definiti<strong>on</strong> of a substance under REACH (see secti<strong>on</strong> 1.3) is very broad and includes not<br />
<strong>on</strong>ly chemicals whether hazardous or not, but every type of substance manufactured in or<br />
imported into the EU. It includes substances which are already closely regulated by other<br />
legislati<strong>on</strong> such as radioactive substances, medicines, food or feedingstuffs, biocides or<br />
pesticides. These substances are completely or partially exempted from REACH or from the<br />
registrati<strong>on</strong> requirements (see following secti<strong>on</strong>s below). Other substances within the scope of<br />
specific pieces of legislati<strong>on</strong>, e.g. food-packaging and cosmetics, although subject to<br />
registrati<strong>on</strong>, have reduced risk assessment requirements under REACH (see secti<strong>on</strong> 3.2.1).<br />
When the registrant manufactures or imports the substance in the nanoform as well as in the<br />
bulk form, the registrati<strong>on</strong> dossier should include the informati<strong>on</strong> of the substance in both the<br />
Annankatu 18, P.O. Box 400, FI-00121 Helsinki, Finland | Tel. +358 9 686180 | Fax +358 9 68618210 | echa.europa.eu