Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<str<strong>on</strong>g>Guidance</str<strong>on</strong>g> <strong>on</strong> registrati<strong>on</strong><br />
Versi<strong>on</strong> 2.0 May 2012 47<br />
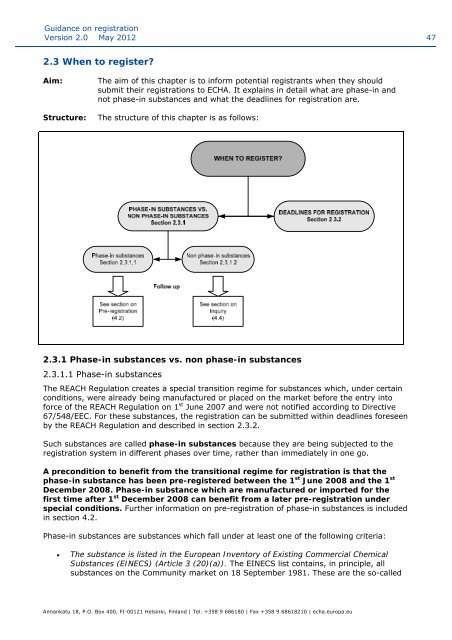
2.3 When to register?<br />
Aim:<br />
Structure:<br />
The aim of this chapter is to inform potential registrants when they should<br />
submit their registrati<strong>on</strong>s to <strong>ECHA</strong>. It explains in detail what are phase-in and<br />
not phase-in substances and what the deadlines for registrati<strong>on</strong> are.<br />
The structure of this chapter is as follows:<br />
2.3.1 Phase-in substances vs. n<strong>on</strong> phase-in substances<br />
2.3.1.1 Phase-in substances<br />
The REACH Regulati<strong>on</strong> creates a special transiti<strong>on</strong> regime for substances which, under certain<br />
c<strong>on</strong>diti<strong>on</strong>s, were already being manufactured or placed <strong>on</strong> the market before the entry into<br />
force of the REACH Regulati<strong>on</strong> <strong>on</strong> 1 st June 2007 and were not notified according to Directive<br />
67/548/EEC. For these substances, the registrati<strong>on</strong> can be submitted within deadlines foreseen<br />
by the REACH Regulati<strong>on</strong> and described in secti<strong>on</strong> 2.3.2.<br />
Such substances are called phase-in substances because they are being subjected to the<br />
registrati<strong>on</strong> system in different phases over time, rather than immediately in <strong>on</strong>e go.<br />
A prec<strong>on</strong>diti<strong>on</strong> to benefit from the transiti<strong>on</strong>al regime for registrati<strong>on</strong> is that the<br />
phase-in substance has been pre-registered between the 1 st June 2008 and the 1 st<br />
December 2008. Phase-in substance which are manufactured or imported for the<br />
first time after 1 st December 2008 can benefit from a later pre-registrati<strong>on</strong> under<br />
special c<strong>on</strong>diti<strong>on</strong>s. Further informati<strong>on</strong> <strong>on</strong> pre-registrati<strong>on</strong> of phase-in substances is included<br />
in secti<strong>on</strong> 4.2.<br />
Phase-in substances are substances which fall under at least <strong>on</strong>e of the following criteria:<br />
<br />
The substance is listed in the European Inventory of Existing Commercial Chemical<br />
Substances (EINECS) (Article 3 (20)(a)). The EINECS list c<strong>on</strong>tains, in principle, all<br />
substances <strong>on</strong> the Community market <strong>on</strong> 18 September 1981. These are the so-called<br />
Annankatu 18, P.O. Box 400, FI-00121 Helsinki, Finland | Tel. +358 9 686180 | Fax +358 9 68618210 | echa.europa.eu