Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<str<strong>on</strong>g>Guidance</str<strong>on</strong>g> <strong>on</strong> registrati<strong>on</strong><br />
Versi<strong>on</strong> 2.0 May 2012 49<br />
For phase-in substances, which are manufactured or imported in a quantity of <strong>on</strong>e t<strong>on</strong>ne or<br />
more per year and which have been pre-registered between 1 June 2008 and 1 December<br />
2008 (inclusive), the registrati<strong>on</strong> provisi<strong>on</strong>s are applied in a stepwise way to facilitate the<br />
transiti<strong>on</strong> to REACH.<br />
The transiti<strong>on</strong>al arrangements introduce different deadlines for registrati<strong>on</strong>, without the need<br />
to interrupt the manufacture or import of these substances.<br />
The deadlines set for the registrati<strong>on</strong> of phase-in substances have been based <strong>on</strong> the t<strong>on</strong>nage<br />
manufactured or imported per manufacturer or importer or producer of articles. This follows<br />
from the assumpti<strong>on</strong> that chemicals manufactured in high volumes will in many cases be more<br />
likely to present a greater risk to humans and the envir<strong>on</strong>ment. A greater priority has also<br />
been given to substances of higher c<strong>on</strong>cern, such as carcinogenic, mutagenic and reprotoxic<br />
substances (CMR) and substances which are very toxic to aquatic organisms and may cause<br />
l<strong>on</strong>g-terms effects in the aquatic envir<strong>on</strong>ment (classified as R50/53).<br />
The 'phase-in' deadlines after entry into force of the Regulati<strong>on</strong> are presented in the<br />
following Table (applicable <strong>on</strong>ly if the substance has been pre-registered).<br />
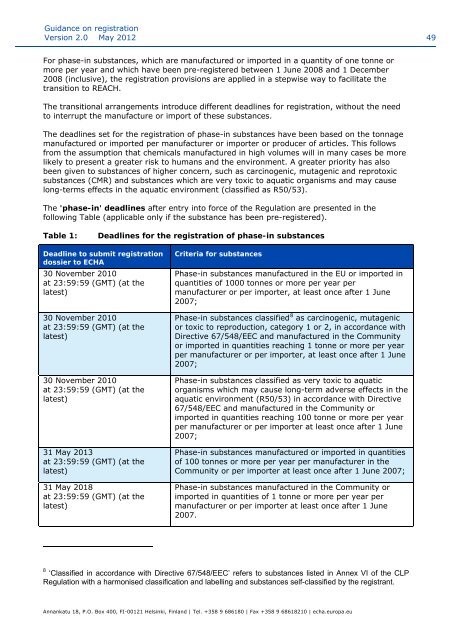
Table 1:<br />
Deadlines for the registrati<strong>on</strong> of phase-in substances<br />
Deadline to submit registrati<strong>on</strong><br />
dossier to <strong>ECHA</strong><br />
30 November 2010<br />
at 23:59:59 (GMT) (at the<br />
latest)<br />
30 November 2010<br />
at 23:59:59 (GMT) (at the<br />
latest)<br />
30 November 2010<br />
at 23:59:59 (GMT) (at the<br />
latest)<br />
31 May 2013<br />
at 23:59:59 (GMT) (at the<br />
latest)<br />
31 May 2018<br />
at 23:59:59 (GMT) (at the<br />
latest)<br />
Criteria for substances<br />
Phase-in substances manufactured in the EU or imported in<br />
quantities of 1000 t<strong>on</strong>nes or more per year per<br />
manufacturer or per importer, at least <strong>on</strong>ce after 1 June<br />
2007;<br />
Phase-in substances classified 8 as carcinogenic, mutagenic<br />
or toxic to reproducti<strong>on</strong>, category 1 or 2, in accordance with<br />
Directive 67/548/EEC and manufactured in the Community<br />
or imported in quantities reaching 1 t<strong>on</strong>ne or more per year<br />
per manufacturer or per importer, at least <strong>on</strong>ce after 1 June<br />
2007;<br />
Phase-in substances classified as very toxic to aquatic<br />
organisms which may cause l<strong>on</strong>g-term adverse effects in the<br />
aquatic envir<strong>on</strong>ment (R50/53) in accordance with Directive<br />
67/548/EEC and manufactured in the Community or<br />
imported in quantities reaching 100 t<strong>on</strong>ne or more per year<br />
per manufacturer or per importer at least <strong>on</strong>ce after 1 June<br />
2007;<br />
Phase-in substances manufactured or imported in quantities<br />
of 100 t<strong>on</strong>nes or more per year per manufacturer in the<br />
Community or per importer at least <strong>on</strong>ce after 1 June 2007;<br />
Phase-in substances manufactured in the Community or<br />
imported in quantities of 1 t<strong>on</strong>ne or more per year per<br />
manufacturer or per importer at least <strong>on</strong>ce after 1 June<br />
2007.<br />
8 ‘Classified in accordance with Directive 67/548/EEC’ refers to substances listed in Annex VI of the CLP<br />
Regulati<strong>on</strong> with a harm<strong>on</strong>ised classificati<strong>on</strong> and labelling and substances self-classified by the registrant.<br />
Annankatu 18, P.O. Box 400, FI-00121 Helsinki, Finland | Tel. +358 9 686180 | Fax +358 9 68618210 | echa.europa.eu