Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
Guidance on registration - ECHA - Europa
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<str<strong>on</strong>g>Guidance</str<strong>on</strong>g> <strong>on</strong> registrati<strong>on</strong><br />
Versi<strong>on</strong> 2.0 May 2012 69<br />
5 Preparati<strong>on</strong> of the registrati<strong>on</strong> dossier<br />
Aim:<br />
The aim of this chapter is to describe how to prepare a registrati<strong>on</strong> dossier. It<br />
offers an overview <strong>on</strong> the informati<strong>on</strong> the registrant has to submit as part of his<br />
registrati<strong>on</strong> dossier and explains how this informati<strong>on</strong> has to be reported. It does<br />
not, however, provide specific practical instructi<strong>on</strong>s <strong>on</strong> how to successfully<br />
submit a registrati<strong>on</strong> dossier to <strong>ECHA</strong>. For this latter informati<strong>on</strong>, the reader is<br />
advised to c<strong>on</strong>sult Part II of this guidance where the different steps to generate<br />
and submit a dossier are described in detail.<br />
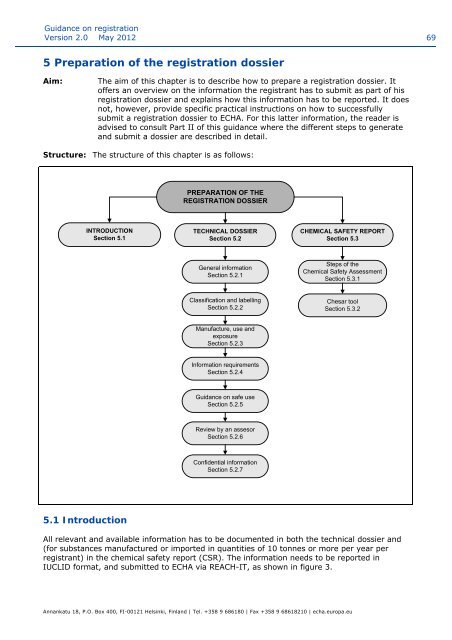
Structure: The structure of this chapter is as follows:<br />
PREPARATION OF THE<br />
REGISTRATION DOSSIER<br />
INTRODUCTION<br />
Secti<strong>on</strong> 5.1<br />
TECHNICAL DOSSIER<br />
Secti<strong>on</strong> 5.2<br />
CHEMICAL SAFETY REPORT<br />
Secti<strong>on</strong> 5.3<br />
General informati<strong>on</strong><br />
Secti<strong>on</strong> 5.2.1<br />
Steps of the<br />
Chemical Safety Assessment<br />
Secti<strong>on</strong> 5.3.1<br />
Classificati<strong>on</strong> and labelling<br />
Secti<strong>on</strong> 5.2.2<br />
Chesar tool<br />
Secti<strong>on</strong> 5.3.2<br />
Manufacture, use and<br />
exposure<br />
Secti<strong>on</strong> 5.2.3<br />
Informati<strong>on</strong> requirements<br />
Secti<strong>on</strong> 5.2.4<br />
<str<strong>on</strong>g>Guidance</str<strong>on</strong>g> <strong>on</strong> safe use<br />
Secti<strong>on</strong> 5.2.5<br />
Review by an assesor<br />
Secti<strong>on</strong> 5.2.6<br />
C<strong>on</strong>fidential informati<strong>on</strong><br />
Secti<strong>on</strong> 5.2.7<br />
5.1 Introducti<strong>on</strong><br />
All relevant and available informati<strong>on</strong> has to be documented in both the technical dossier and<br />
(for substances manufactured or imported in quantities of 10 t<strong>on</strong>nes or more per year per<br />
registrant) in the chemical safety report (CSR). The informati<strong>on</strong> needs to be reported in<br />
IUCLID format, and submitted to <strong>ECHA</strong> via REACH-IT, as shown in figure 3.<br />
Annankatu 18, P.O. Box 400, FI-00121 Helsinki, Finland | Tel. +358 9 686180 | Fax +358 9 68618210 | echa.europa.eu