Principles of cell signaling - UT Southwestern
Principles of cell signaling - UT Southwestern
Principles of cell signaling - UT Southwestern
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
39057_ch14_<strong>cell</strong>bio.qxd 8/28/06 5:11 PM Page 620<br />
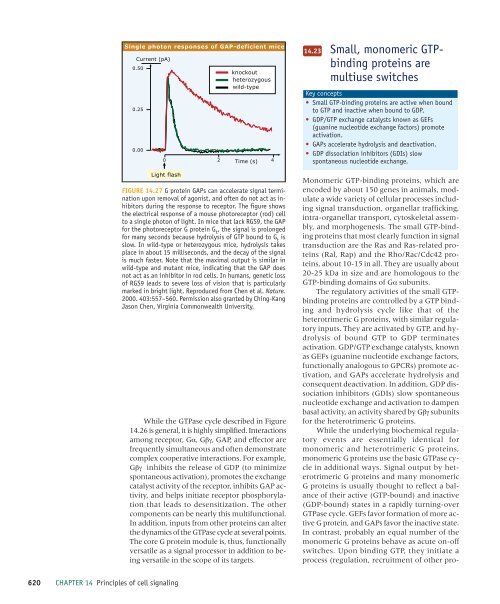
Single photon responses <strong>of</strong> GAP-deficient mice<br />
Current (pA)<br />
0.50<br />
0.25<br />
0.00<br />
knockout<br />
heterozygous<br />
wild-type<br />
0 2 Time (s) 4<br />
Light flash<br />
FIGURE 14.27 G protein GAPs can accelerate signal termination<br />
upon removal <strong>of</strong> agonist, and <strong>of</strong>ten do not act as inhibitors<br />
during the response to receptor. The figure shows<br />
the electrical response <strong>of</strong> a mouse photoreceptor (rod) <strong>cell</strong><br />
to a single photon <strong>of</strong> light. In mice that lack RGS9, the GAP<br />
for the photoreceptor G protein G t<br />
, the signal is prolonged<br />
for many seconds because hydrolysis <strong>of</strong> GTP bound to G t<br />
is<br />
slow. In wild-type or heterozygous mice, hydrolysis takes<br />
place in about 15 milliseconds, and the decay <strong>of</strong> the signal<br />
is much faster. Note that the maximal output is similar in<br />
wild-type and mutant mice, indicating that the GAP does<br />
not act as an inhibitor in rod <strong>cell</strong>s. In humans, genetic loss<br />
<strong>of</strong> RGS9 leads to severe loss <strong>of</strong> vision that is particularly<br />
marked in bright light. Reproduced from Chen et al. Nature.<br />
2000. 403:557–560. Permission also granted by Ching-Kang<br />
Jason Chen, Virginia Commonwealth University.<br />
While the GTPase cycle described in Figure<br />
14.26 is general, it is highly simplified. Interactions<br />
among receptor, Gα, Gβγ, GAP, and effector are<br />
frequently simultaneous and <strong>of</strong>ten demonstrate<br />
complex cooperative interactions. For example,<br />
Gβγ inhibits the release <strong>of</strong> GDP (to minimize<br />
spontaneous activation), promotes the exchange<br />
catalyst activity <strong>of</strong> the receptor, inhibits GAP activity,<br />
and helps initiate receptor phosphorylation<br />
that leads to desensitization. The other<br />
components can be nearly this multifunctional.<br />
In addition, inputs from other proteins can alter<br />
the dynamics <strong>of</strong> the GTPase cycle at several points.<br />
The core G protein module is, thus, functionally<br />
versatile as a signal processor in addition to being<br />
versatile in the scope <strong>of</strong> its targets.<br />
14.23<br />
Small, monomeric GTPbinding<br />
proteins are<br />
multiuse switches<br />
Key concepts<br />
• Small GTP-binding proteins are active when bound<br />
to GTP and inactive when bound to GDP.<br />
• GDP/GTP exchange catalysts known as GEFs<br />
(guanine nucleotide exchange factors) promote<br />
activation.<br />
• GAPs accelerate hydrolysis and deactivation.<br />
• GDP dissociation inhibitors (GDIs) slow<br />
spontaneous nucleotide exchange.<br />
Monomeric GTP-binding proteins, which are<br />
encoded by about 150 genes in animals, modulate<br />
a wide variety <strong>of</strong> <strong>cell</strong>ular processes including<br />
signal transduction, organellar trafficking,<br />
intra-organellar transport, cytoskeletal assembly,<br />
and morphogenesis. The small GTP-binding<br />
proteins that most clearly function in signal<br />
transduction are the Ras and Ras-related proteins<br />
(Ral, Rap) and the Rho/Rac/Cdc42 proteins,<br />
about 10-15 in all. They are usually about<br />
20-25 kDa in size and are homologous to the<br />
GTP-binding domains <strong>of</strong> Gα subunits.<br />
The regulatory activities <strong>of</strong> the small GTPbinding<br />
proteins are controlled by a GTP binding<br />
and hydrolysis cycle like that <strong>of</strong> the<br />
heterotrimeric G proteins, with similar regulatory<br />
inputs. They are activated by GTP, and hydrolysis<br />
<strong>of</strong> bound GTP to GDP terminates<br />
activation. GDP/GTP exchange catalysts, known<br />
as GEFs (guanine nucleotide exchange factors,<br />
functionally analogous to GPCRs) promote activation,<br />
and GAPs accelerate hydrolysis and<br />
consequent deactivation. In addition, GDP dissociation<br />
inhibitors (GDIs) slow spontaneous<br />
nucleotide exchange and activation to dampen<br />
basal activity, an activity shared by Gβγ subunits<br />
for the heterotrimeric G proteins.<br />
While the underlying biochemical regulatory<br />
events are essentially identical for<br />
monomeric and heterotrimeric G proteins,<br />
monomeric G proteins use the basic GTPase cycle<br />
in additional ways. Signal output by heterotrimeric<br />
G proteins and many monomeric<br />
G proteins is usually thought to reflect a balance<br />
<strong>of</strong> their active (GTP-bound) and inactive<br />
(GDP-bound) states in a rapidly turning-over<br />
GTPase cycle. GEFs favor formation <strong>of</strong> more active<br />
G protein, and GAPs favor the inactive state.<br />
In contrast, probably an equal number <strong>of</strong> the<br />
monomeric G proteins behave as acute on-<strong>of</strong>f<br />
switches. Upon binding GTP, they initiate a<br />
process (regulation, recruitment <strong>of</strong> other pro-<br />
620 CHAPTER 14 <strong>Principles</strong> <strong>of</strong> <strong>cell</strong> <strong>signaling</strong>