Principles of cell signaling - UT Southwestern
Principles of cell signaling - UT Southwestern
Principles of cell signaling - UT Southwestern
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
39057_ch14_<strong>cell</strong>bio.qxd 8/28/06 5:11 PM Page 624<br />
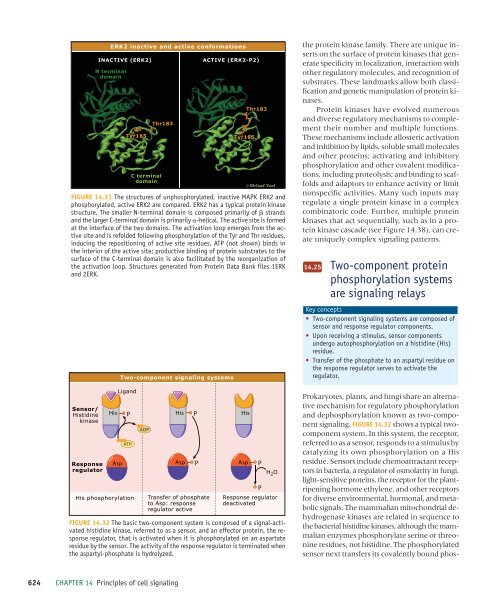
ERK2 inactive and active conformations<br />
INACTIVE (ERK2)<br />
N terminal<br />
domain<br />
Tyr185<br />
Thr183<br />
C terminal<br />
domain<br />
ACTIVE (ERK2-P2)<br />
Tyr185<br />
Thr183<br />
FIGURE 14.31 The structures <strong>of</strong> unphosphorylated, inactive MAPK ERK2 and<br />
phosphorylated, active ERK2 are compared. ERK2 has a typical protein kinase<br />
structure. The smaller N-terminal domain is composed primarily <strong>of</strong> strands<br />
and the larger C-terminal domain is primarily -helical. The active site is formed<br />
at the interface <strong>of</strong> the two domains. The activation loop emerges from the active<br />
site and is refolded following phosphorylation <strong>of</strong> the Tyr and Thr residues,<br />
inducing the repositioning <strong>of</strong> active site residues. ATP (not shown) binds in<br />
the interior <strong>of</strong> the active site; productive binding <strong>of</strong> protein substrates to the<br />
surface <strong>of</strong> the C-terminal domain is also facilitated by the reorganization <strong>of</strong><br />
the activation loop. Structures generated from Protein Data Bank files 1ERK<br />
and 2ERK.<br />
Sensor/<br />
Histidine<br />
kinase<br />
Response<br />
regulator<br />
His<br />
Asp<br />
Ligand<br />
P<br />
ATP<br />
His phosphorylation<br />
Two-component <strong>signaling</strong> systems<br />
ADP<br />
His<br />
Asp<br />
P<br />
P<br />
Transfer <strong>of</strong> phosphate<br />
to Asp: response<br />
regulator active<br />
His<br />
Asp<br />
P<br />
P<br />
H 2 O<br />
Response regulator<br />
deactivated<br />
FIGURE 14.32 The basic two-component system is composed <strong>of</strong> a signal-activated<br />
histidine kinase, referred to as a sensor, and an effector protein, the response<br />
regulator, that is activated when it is phosphorylated on an aspartate<br />
residue by the sensor. The activity <strong>of</strong> the response regulator is terminated when<br />
the aspartyl-phosphate is hydrolyzed.<br />
the protein kinase family. There are unique inserts<br />
on the surface <strong>of</strong> protein kinases that generate<br />
specificity in localization, interaction with<br />
other regulatory molecules, and recognition <strong>of</strong><br />
substrates. These landmarks allow both classification<br />
and genetic manipulation <strong>of</strong> protein kinases.<br />
Protein kinases have evolved numerous<br />
and diverse regulatory mechanisms to complement<br />
their number and multiple functions.<br />
These mechanisms include allosteric activation<br />
and inhibition by lipids, soluble small molecules<br />
and other proteins; activating and inhibitory<br />
phosphorylation and other covalent modifications,<br />
including proteolysis; and binding to scaffolds<br />
and adaptors to enhance activity or limit<br />
nonspecific activities. Many such inputs may<br />
regulate a single protein kinase in a complex<br />
combinatoric code. Further, multiple protein<br />
kinases that act sequentially, such as in a protein<br />
kinase cascade (see Figure 14.38), can create<br />
uniquely complex <strong>signaling</strong> patterns.<br />
14.25<br />
Two-component protein<br />
phosphorylation systems<br />
are <strong>signaling</strong> relays<br />
Key concepts<br />
• Two-component <strong>signaling</strong> systems are composed <strong>of</strong><br />
sensor and response regulator components.<br />
• Upon receiving a stimulus, sensor components<br />
undergo autophosphorylation on a histidine (His)<br />
residue.<br />
• Transfer <strong>of</strong> the phosphate to an aspartyl residue on<br />
the response regulator serves to activate the<br />
regulator.<br />
Prokaryotes, plants, and fungi share an alternative<br />
mechanism for regulatory phosphorylation<br />
and dephosphorylation known as two-component<br />
<strong>signaling</strong>. FIGURE 14.32 shows a typical twocomponent<br />
system. In this system, the receptor,<br />
referred to as a sensor, responds to a stimulus by<br />
catalyzing its own phosphorylation on a His<br />
residue. Sensors include chemoattractant receptors<br />
in bacteria, a regulator <strong>of</strong> osmolarity in fungi,<br />
light-sensitive proteins, the receptor for the plantripening<br />
hormone ethylene, and other receptors<br />
for diverse environmental, hormonal, and metabolic<br />
signals. The mammalian mitochondrial dehydrogenase<br />
kinases are related in sequence to<br />
the bacterial histidine kinases, although the mammalian<br />
enzymes phosphorylate serine or threonine<br />
residues, not histidine. The phosphorylated<br />
sensor next transfers its covalently bound phos-<br />
624 CHAPTER 14 <strong>Principles</strong> <strong>of</strong> <strong>cell</strong> <strong>signaling</strong>