Principles of cell signaling - UT Southwestern
Principles of cell signaling - UT Southwestern
Principles of cell signaling - UT Southwestern
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
39057_ch14_<strong>cell</strong>bio.qxd 8/28/06 5:11 PM Page 634<br />
Crk<br />
ECM<br />
Crk<br />
Src<br />
CAS<br />
CYTOPLASM<br />
FAK<br />
SOS<br />
Integrin <strong>signaling</strong><br />
INTEGRINS<br />
Paxillin<br />
Talin<br />
Vinculin<br />
Grb2<br />
Actin<br />
filament<br />
ILK<br />
p85 PI 3-<br />
Kinase<br />
p110<br />
Vinculin<br />
Tensin<br />
Talin<br />
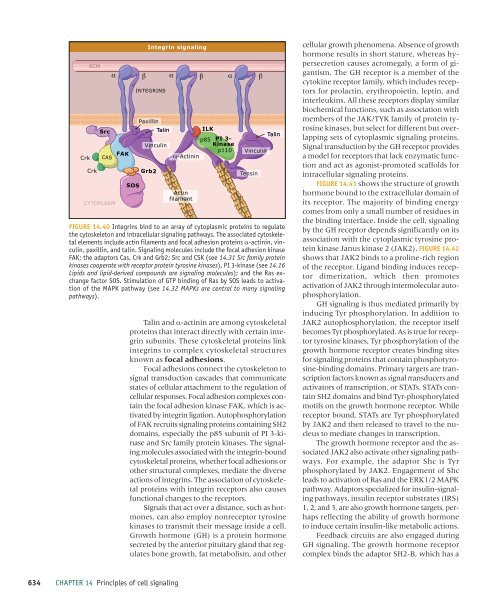
FIGURE 14.40 Integrins bind to an array <strong>of</strong> cytoplasmic proteins to regulate<br />
the cytoskeleton and intra<strong>cell</strong>ular <strong>signaling</strong> pathways. The associated cytoskeletal<br />
elements include actin filaments and focal adhesion proteins -actinin, vinculin,<br />
paxillin, and talin. Signaling molecules include the focal adhesion kinase<br />
FAK; the adaptors Cas, Crk and Grb2; Src and CSK (see 14.31 Src family protein<br />
kinases cooperate with receptor protein tyrosine kinases), PI 3-kinase (see 14.16<br />
Lipids and lipid-derived compounds are <strong>signaling</strong> molecules); and the Ras exchange<br />
factor SOS. Stimulation <strong>of</strong> GTP binding <strong>of</strong> Ras by SOS leads to activation<br />
<strong>of</strong> the MAPK pathway (see 14.32 MAPKs are central to many <strong>signaling</strong><br />
pathways).<br />
Talin and α-actinin are among cytoskeletal<br />
proteins that interact directly with certain integrin<br />
subunits. These cytoskeletal proteins link<br />
integrins to complex cytoskeletal structures<br />
known as focal adhesions.<br />
Focal adhesions connect the cytoskeleton to<br />
signal transduction cascades that communicate<br />
states <strong>of</strong> <strong>cell</strong>ular attachment to the regulation <strong>of</strong><br />
<strong>cell</strong>ular responses. Focal adhesion complexes contain<br />
the focal adhesion kinase FAK, which is activated<br />
by integrin ligation. Autophosphorylation<br />
<strong>of</strong> FAK recruits <strong>signaling</strong> proteins containing SH2<br />
domains, especially the p85 subunit <strong>of</strong> PI 3-kinase<br />
and Src family protein kinases. The <strong>signaling</strong><br />
molecules associated with the integrin-bound<br />
cytoskeletal proteins, whether focal adhesions or<br />
other structural complexes, mediate the diverse<br />
actions <strong>of</strong> integrins. The association <strong>of</strong> cytoskeletal<br />
proteins with integrin receptors also causes<br />
functional changes to the receptors.<br />
Signals that act over a distance, such as hormones,<br />
can also employ nonreceptor tyrosine<br />
kinases to transmit their message inside a <strong>cell</strong>.<br />
Growth hormone (GH) is a protein hormone<br />
secreted by the anterior pituitary gland that regulates<br />
bone growth, fat metabolism, and other<br />
<strong>cell</strong>ular growth phenomena. Absence <strong>of</strong> growth<br />
hormone results in short stature, whereas hypersecretion<br />
causes acromegaly, a form <strong>of</strong> gigantism.<br />
The GH receptor is a member <strong>of</strong> the<br />
cytokine receptor family, which includes receptors<br />
for prolactin, erythropoietin, leptin, and<br />
interleukins. All these receptors display similar<br />
biochemical functions, such as association with<br />
members <strong>of</strong> the JAK/TYK family <strong>of</strong> protein tyrosine<br />
kinases, but select for different but overlapping<br />
sets <strong>of</strong> cytoplasmic <strong>signaling</strong> proteins.<br />
Signal transduction by the GH receptor provides<br />
a model for receptors that lack enzymatic function<br />
and act as agonist-promoted scaffolds for<br />
intra<strong>cell</strong>ular <strong>signaling</strong> proteins.<br />
FIGURE 14.41 shows the structure <strong>of</strong> growth<br />
hormone bound to the extra<strong>cell</strong>ular domain <strong>of</strong><br />
its receptor. The majority <strong>of</strong> binding energy<br />
comes from only a small number <strong>of</strong> residues in<br />
the binding interface. Inside the <strong>cell</strong>, <strong>signaling</strong><br />
by the GH receptor depends significantly on its<br />
association with the cytoplasmic tyrosine protein<br />
kinase Janus kinase 2 (JAK2). FIGURE 14.42<br />
shows that JAK2 binds to a proline-rich region<br />
<strong>of</strong> the receptor. Ligand binding induces receptor<br />
dimerization, which then promotes<br />
activation <strong>of</strong> JAK2 through intermolecular autophosphorylation.<br />
GH <strong>signaling</strong> is thus mediated primarily by<br />
inducing Tyr phosphorylation. In addition to<br />
JAK2 autophosphorylation, the receptor itself<br />
becomes Tyr phosphorylated. As is true for receptor<br />
tyrosine kinases, Tyr phosphorylation <strong>of</strong> the<br />
growth hormone receptor creates binding sites<br />
for <strong>signaling</strong> proteins that contain phosphotyrosine-binding<br />
domains. Primary targets are transcription<br />
factors known as signal transducers and<br />
activators <strong>of</strong> transcription, or STATs. STATs contain<br />
SH2 domains and bind Tyr-phosphorylated<br />
motifs on the growth hormone receptor. While<br />
receptor bound, STATs are Tyr phosphorylated<br />
by JAK2 and then released to travel to the nucleus<br />
to mediate changes in transcription.<br />
The growth hormone receptor and the associated<br />
JAK2 also activate other <strong>signaling</strong> pathways.<br />
For example, the adaptor Shc is Tyr<br />
phosphorylated by JAK2. Engagement <strong>of</strong> Shc<br />
leads to activation <strong>of</strong> Ras and the ERK1/2 MAPK<br />
pathway. Adaptors specialized for insulin-<strong>signaling</strong><br />
pathways, insulin receptor substrates (IRS)<br />
1, 2, and 3, are also growth hormone targets, perhaps<br />
reflecting the ability <strong>of</strong> growth hormone<br />
to induce certain insulin-like metabolic actions.<br />
Feedback circuits are also engaged during<br />
GH <strong>signaling</strong>. The growth hormone receptor<br />
complex binds the adaptor SH2-B, which has a<br />
634 CHAPTER 14 <strong>Principles</strong> <strong>of</strong> <strong>cell</strong> <strong>signaling</strong>