Book of Abstracts - Ruhr-Universität Bochum
Book of Abstracts - Ruhr-Universität Bochum
Book of Abstracts - Ruhr-Universität Bochum
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
P-56<br />
ISBOMC `10 5.7 – 9.7. 2010 <strong>Ruhr</strong>-<strong>Universität</strong> <strong>Bochum</strong><br />
Novel MRI Contrast Agents based on a Silsesquioxane Core<br />
Jörg Henig, a,b* É. Jakab-Tóth, c J. Engelmann, d S. Gottschalk, d H.A. Mayer a<br />
a <strong>Universität</strong> Tübingen, Institut für Anorganische Chemie, Auf der Morgenstelle 18, 72076 Tübingen,<br />
Germany. b <strong>Ruhr</strong>-<strong>Universität</strong> <strong>Bochum</strong>, Zentrum für Elektrochemie, <strong>Universität</strong>sstraße 150, 44801<br />
<strong>Bochum</strong>, Germany. c CNRS Orléans, Le Centre de Biophysique Moléculaire, 45071 Orléans, France.<br />
d Max-Planck-Institut für biologische Kybernetik, Hochfeld-Magnetresonanz-Zentrum, 72076<br />
Tübingen, Germany. E-mail: joerg.henig@rub.de<br />
Large macromolecular MRI contrast agents (CAs) bearing several paramagnetic gadolinium(III)<br />
centres show usually significantly higher relaxivities than small size CAs and are particularly useful<br />
for target specific imaging. However, the large size slows down the excretion rate <strong>of</strong> the CA, which is<br />
a major limitation for clinical use. Furthermore, the Solomon-Bloembergen-Morgan theory predicts<br />
that at the high magnetic fields <strong>of</strong> modern clinical and especially research MRI scanners, medium-size<br />
CAs rather than very large systems are the most favored in order to achieve high relaxivities. Here we<br />
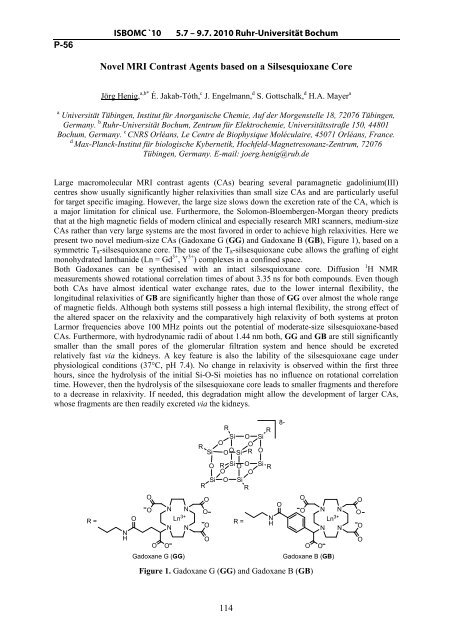
present two novel medium-size CAs (Gadoxane G (GG) and Gadoxane B (GB), Figure 1), based on a<br />
symmetric T8-silsesquioxane core. The use <strong>of</strong> the T8-silsesquioxane cube allows the grafting <strong>of</strong> eight<br />
monohydrated lanthanide (Ln = Gd 3+ , Y 3+ ) complexes in a confined space.<br />
Both Gadoxanes can be synthesised with an intact silsesquioxane core. Diffusion 1 H NMR<br />
measurements showed rotational correlation times <strong>of</strong> about 3.35 ns for both compounds. Even though<br />
both CAs have almost identical water exchange rates, due to the lower internal flexibility, the<br />
longitudinal relaxivities <strong>of</strong> GB are significantly higher than those <strong>of</strong> GG over almost the whole range<br />
<strong>of</strong> magnetic fields. Although both systems still possess a high internal flexibility, the strong effect <strong>of</strong><br />
the altered spacer on the relaxivity and the comparatively high relaxivity <strong>of</strong> both systems at proton<br />
Larmor frequencies above 100 MHz points out the potential <strong>of</strong> moderate-size silsesquioxane-based<br />
CAs. Furthermore, with hydrodynamic radii <strong>of</strong> about 1.44 nm both, GG and GB are still significantly<br />
smaller than the small pores <strong>of</strong> the glomerular filtration system and hence should be excreted<br />
relatively fast via the kidneys. A key feature is also the lability <strong>of</strong> the silsesquioxane cage under<br />
physiological conditions (37°C, pH 7.4). No change in relaxivity is observed within the first three<br />
hours, since the hydrolysis <strong>of</strong> the initial Si-O-Si moieties has no influence on rotational correlation<br />
time. However, then the hydrolysis <strong>of</strong> the silsesquioxane core leads to smaller fragments and therefore<br />
to a decrease in relaxivity. If needed, this degradation might allow the development <strong>of</strong> larger CAs,<br />
whose fragments are then readily excreted via the kidneys.<br />
R =<br />
N<br />
H<br />
O<br />
O<br />
O<br />
N N<br />
N<br />
O O<br />
Ln 3+<br />
Gadoxane G (GG)<br />
N<br />
R<br />
R<br />
R<br />
Si<br />
Si O<br />
O O<br />
OO Si R<br />
Si<br />
O<br />
O R<br />
O<br />
Si O O<br />
O<br />
Si<br />
R<br />
R<br />
Si O Si<br />
R<br />
O<br />
O<br />
O<br />
O<br />
114<br />
R =<br />
N<br />
H<br />
8-<br />
O<br />
O<br />
O<br />
N N<br />
N<br />
O O<br />
Ln 3+<br />
Gadoxane B (GB)<br />
Figure 1. Gadoxane G (GG) and Gadoxane B (GB)<br />
N<br />
O<br />
O<br />
O<br />
O