n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
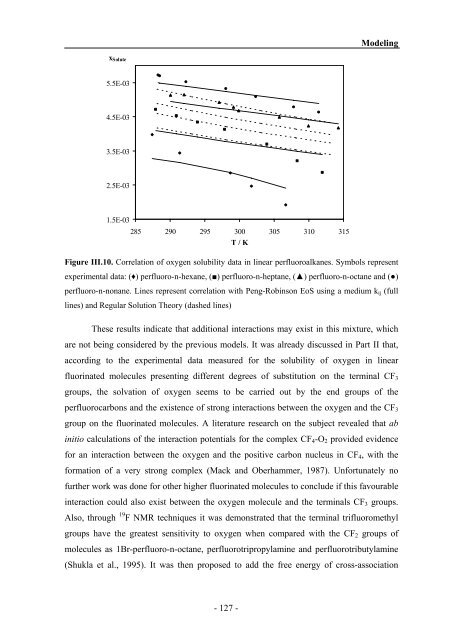
xSolute<br />
5.5E-03<br />
4.5E-03<br />
3.5E-03<br />
2.5E-03<br />
1.5E-03<br />
285 290 295 300 305 310 315<br />
T / K<br />
Modeling<br />
Figure III.10. Correlation of oxygen solubility data in linear perfluoroalkanes. Symbols represent<br />
experimental data: (♦) perfluoro-n-hexane, (■) perfluoro-n-heptane, (▲) perfluoro-n-octane <strong>and</strong> (●)<br />
perfluoro-n-nonane. Lines represent correlation with Peng-Robinson EoS using a medium kij (full<br />
lines) <strong>and</strong> Regular Solution Theory (dashed lines)<br />
These results indicate that additional interactions may exist in this mixture, which<br />
are not being considered by the previous models. It was already discussed in Part II that,<br />
according to the experimental data measured for the solubility of oxygen in linear<br />
fluorinated molecules presenting different degrees of substitution on the terminal CF3<br />
groups, the solvation of oxygen seems to be carried out by the end groups of the<br />
perfluorocarbons <strong>and</strong> the existence of strong interactions between the oxygen <strong>and</strong> the CF3<br />
group on the fluorinated molecules. A literature <strong>research</strong> on the subject revealed that ab<br />
initio calculations of the interaction potentials for the complex CF4-O2 provided evidence<br />
for an interaction between the oxygen <strong>and</strong> the positive carbon nucleus in CF4, with the<br />
formation of a very strong complex (Mack <strong>and</strong> Oberhammer, 1987). Unfortunately no<br />
further work was done for other higher fluorinated molecules to conclude if this favourable<br />
interaction could also exist between the oxygen molecule <strong>and</strong> the terminals CF3 groups.<br />
Also, through 19 F NMR techniques it was demonstrated that the terminal trifluoromethyl<br />
groups have the greatest sensitivity to oxygen when compared with the CF2 groups of<br />
molecules as 1Br-perfluoro-n-octane, perfluorotripropylamine <strong>and</strong> perfluorotributylamine<br />
(Shukla et al., 1995). It was then proposed to add the free energy of cross-association<br />
- 127 -