- Page 1 and 2:
Ana Maria Antunes Dias Universidade
- Page 3 and 4:
o júri presidente Prof. Dr. Joaqui
- Page 5 and 6:
palavras-chave resumo perfluoroalca
- Page 7 and 8:
Contents Notation List of Tables Li
- Page 9 and 10:
Notation Abbreviations AAD EoS LCST
- Page 11 and 12:
List of Tables Table I.1 Average Bo
- Page 13 and 14:
Table III.6 Adjusted Binary Paramet
- Page 15 and 16:
Figure II.9 Comparison between corr
- Page 17 and 18:
Figure III.8 Temperature-density di
- Page 19 and 20:
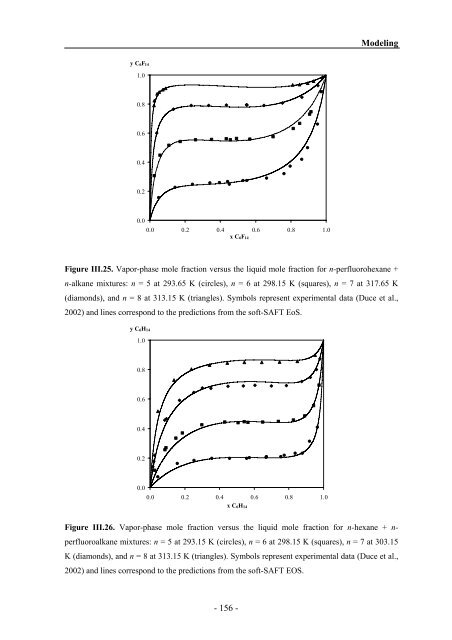
Figure III.25 Vapor-phase mole frac
- Page 21 and 22:
I.1. Fluorine Properties General In
- Page 23 and 24:
Table I.2. Physicochemical Properti
- Page 25 and 26:
General Introduction order to compa
- Page 27 and 28:
General Introduction the numerous a
- Page 29 and 30:
General Introduction carbon dioxide
- Page 31 and 32:
General Introduction animals. That
- Page 33 and 34:
General Introduction Table I.3. Lit
- Page 35 and 36:
References General Introduction Ban
- Page 37 and 38:
General Introduction Hildebrand, J.
- Page 39 and 40:
General Introduction Rowinsky EK. N
- Page 41 and 42:
II. Part EXPERIMENTAL METHODS, RESU
- Page 43 and 44:
Experimental Methods, Results and D
- Page 45 and 46:
Experimental Methods, Results and D
- Page 47 and 48:
Experimental Methods, Results and D
- Page 49 and 50:
Table II.3. (continued) T K ρexp g
- Page 51 and 52:
ρ / g.cm-3 1.750 1.730 1.710 1.690
- Page 53 and 54:
Experimental Methods, Results and D
- Page 55 and 56:
I. 3. Vapour pressure I.3.1. Biblio
- Page 57 and 58:
I.3.2. Apparatus and Procedure Expe
- Page 59 and 60:
I.3.3. Experimental Results and Dis
- Page 61 and 62:
Experimental Methods, Results and D
- Page 63 and 64:
Table II.8. (continued) T K Pexp kP
- Page 65 and 66:
Experimental Methods, Results and D
- Page 67 and 68:
ΔH vap = TΔS vap 2⎛ d ln P ⎞
- Page 69 and 70:
I.4. Solubility at atmospheric pres
- Page 71 and 72:
Experimental Methods, Results and D
- Page 73 and 74:
Experimental Methods, Results and D
- Page 75 and 76:
Experimental Methods, Results and D
- Page 77 and 78:
Experimental Methods, Results and D
- Page 79 and 80:
x2 (T,P2) 7.0E-03 6.0E-03 5.0E-03 4
- Page 81 and 82:
L 2,1 0.70 0.60 0.50 0.40 0.30 285
- Page 83 and 84:
Experimental Methods, Results and D
- Page 85 and 86:
Experimental Methods, Results and D
- Page 87 and 88:
Experimental Methods, Results and D
- Page 89 and 90:
Experimental Methods, Results and D
- Page 91 and 92:
Experimental Methods, Results and D
- Page 93 and 94:
P / MPa 6 5 4 3 2 1 0 P / MPa 14 12
- Page 95 and 96:
II.6. Liquid - Liquid Equilibrium I
- Page 97 and 98:
Experimental Methods, Results and D
- Page 99 and 100:
Experimental Methods, Results and D
- Page 101 and 102:
0 τ β Δ1 2Δ1 [ 1+ B τ + B τ +
- Page 103 and 104:
References Experimental Methods, Re
- Page 105 and 106:
Experimental Methods, Results and D
- Page 107 and 108:
Experimental Methods, Results and D
- Page 109 and 110:
Experimental Methods, Results and D
- Page 111 and 112:
III.1. Introduction Modeling Most c
- Page 113 and 114:
Modeling The pioneering work of Wer
- Page 115 and 116:
III.2. Soft-SAFT Model Modeling A S
- Page 117 and 118:
Modeling The equation of state is w
- Page 119 and 120:
Chain Term Modeling Originally Wert
- Page 121 and 122:
Modeling The model is easily extend
- Page 123 and 124: ( ) ( ) ∑∑∑ 3 2 2 2 2 qq 32π
- Page 125 and 126: Modeling HRT is a promising theory,
- Page 127 and 128: ( ρ) ρ , 0 ≤ ρ ( ρ) 2 Modelin
- Page 129 and 130: III.3. Application to Pure Compound
- Page 131 and 132: Modeling From the optimised paramet
- Page 133 and 134: T / K 400 350 300 250 200 150 100 5
- Page 135 and 136: ln Pvap 1.0E+01 1.0E+00 1.0E-01 1.0
- Page 137 and 138: Modeling Table III.2. Absolute Aver
- Page 139 and 140: Modeling The correlation coefficien
- Page 141 and 142: Pvap (MPa) 4.00 3.50 3.00 2.50 2.00
- Page 143 and 144: Modeling The mixture parameters a a
- Page 145 and 146: Modeling the assumptions made by th
- Page 147 and 148: Modeling between oxygen and perfluo
- Page 149 and 150: xSolute 5.5E-03 5.0E-03 4.5E-03 4.0
- Page 151 and 152: Modeling previous work, dealing wit
- Page 153 and 154: Modeling These results confirm that
- Page 155 and 156: Modeling CO2 binary mixtures using
- Page 157 and 158: Modeling average deviation (AAD) be
- Page 159 and 160: P / MPa 14 12 10 8 6 4 2 0 0 0.2 0.
- Page 161 and 162: Modeling Figures III.16, III.17 and
- Page 163 and 164: Modeling calculations from the orig
- Page 165 and 166: Table III.9. References for VLE exp
- Page 167 and 168: P / MPa 20 18 16 14 12 10 8 6 4 2 0
- Page 169 and 170: Modeling Finally, Figure III.24 pre
- Page 171 and 172: III.4.4. VLE and LLE of Alkane and
- Page 173: Modeling number of perfluro-n-alkan
- Page 177 and 178: P / MPa 0.08 0.07 0.06 0.05 0.04 0.
- Page 179 and 180: P / MPa 0.12 0.10 0.08 0.06 0.04 0.
- Page 181 and 182: Modeling approach based on the meth
- Page 183 and 184: Modeling Blas, F. J.; Vega, L. F.,
- Page 185 and 186: DIPPR, Thermophysical Properties Da
- Page 187 and 188: Modeling Hildebrand, J. H.; Fisher,
- Page 189 and 190: Modeling McCabe, C.; Jackson, SAFT-
- Page 191 and 192: Modeling Poling, B.; Prauznitz, J.;
- Page 193 and 194: Modeling Wertheim, M. S., Fluids wi