n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Modeling<br />
before for the fluorinated compounds. Figures III.20 <strong>and</strong> III.21 present the results obtained<br />
for the CO2/benzene <strong>and</strong> CO2/toluene mixtures.<br />
P / MPa<br />
18<br />
16<br />
14<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
0 0.2 0.4 0.6 0.8 1<br />
x, y CO2<br />
P / MPa<br />
18<br />
16<br />
14<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
0 0.2 0.4 0.6 0.8 1<br />
x, y CO2<br />
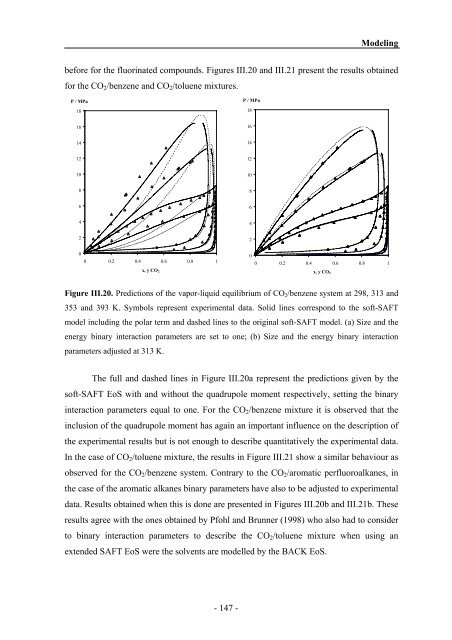
Figure III.20. Predictions of the vapor-liquid equilibrium of CO2/benzene system at 298, 313 <strong>and</strong><br />
353 <strong>and</strong> 393 K. Symbols represent experimental data. Solid lines correspond to the soft-SAFT<br />
model including the polar term <strong>and</strong> dashed lines to the original soft-SAFT model. (a) Size <strong>and</strong> the<br />
energy binary interaction parameters are set to one; (b) Size <strong>and</strong> the energy binary interaction<br />
parameters adjusted at 313 K.<br />
The full <strong>and</strong> dashed lines in Figure III.20a represent the predictions given by the<br />
soft-SAFT EoS with <strong>and</strong> without the quadrupole moment respectively, setting the binary<br />
interaction parameters equal to one. For the CO2/benzene mixture it is observed that the<br />
inclusion of the quadrupole moment has again an important influence on the description of<br />
the experimental results but is not enough to describe quantitatively the experimental data.<br />
In the case of CO2/toluene mixture, the results in Figure III.21 show a similar behaviour as<br />
observed for the CO2/benzene system. Contrary to the CO2/aromatic perfluoroalkanes, in<br />
the case of the aromatic alkanes binary parameters have also to be adjusted to experimental<br />
data. Results obtained when this is done are presented in Figures III.20b <strong>and</strong> III.21b. These<br />
results agree with the ones obtained by Pfohl <strong>and</strong> Brunner (1998) who also had to consider<br />
to binary interaction parameters to describe the CO2/toluene mixture when using an<br />
extended SAFT EoS were the solvents are modelled by the BACK EoS.<br />
- 147 -