n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Modeling<br />
average deviation (AAD) between experimental liquid coexisting densities <strong>and</strong> vapor<br />
pressures of carbon dioxide, perfluorobenzene <strong>and</strong> perfluorotoluene given by the soft-<br />
SAFT EoS, with <strong>and</strong> without the inclusion of the quadrupole moment on the molecule. In<br />
both cases, vapour pressures <strong>and</strong> liquid densities are fitted to experimental data with an<br />
AAD inferior to 3 % <strong>and</strong> 0.5 % respectively. For perfluoro-n-octane <strong>and</strong><br />
perfluoromethylcyclohexane the parameters that will be used are in Table III.1.<br />
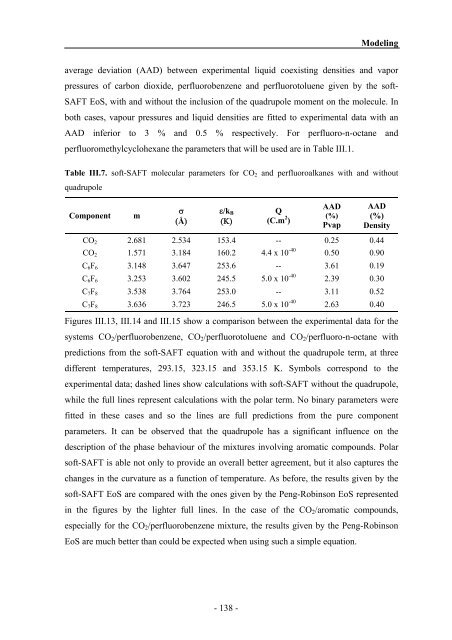
Table III.7. soft-SAFT molecular parameters for CO2 <strong>and</strong> perfluoroalkanes with <strong>and</strong> without<br />
quadrupole<br />
Component m<br />
σ<br />
(Å)<br />
ε/kBB<br />
(Κ)<br />
Q<br />
(C.m 2 )<br />
AAD<br />
(%)<br />
Pvap<br />
AAD<br />
(%)<br />
Density<br />
CO2 2.681 2.534 153.4 -- 0.25 0.44<br />
CO2 1.571 3.184 160.2 4.4 x 10 -40<br />
0.50 0.90<br />
C6F6 3.148 3.647 253.6 -- 3.61 0.19<br />
C6F6 3.253 3.602 245.5 5.0 x 10 -40<br />
2.39 0.30<br />
C7F8 3.538 3.764 253.0 -- 3.11 0.52<br />
5.0 x 10 -40<br />
C7F8 3.636 3.723 246.5 2.63 0.40<br />
Figures III.13, III.14 <strong>and</strong> III.15 show a comparison between the experimental data for the<br />
systems CO2/perfluorobenzene, CO2/perfluorotoluene <strong>and</strong> CO2/perfluoro-n-octane with<br />
predictions from the soft-SAFT equation with <strong>and</strong> without the quadrupole term, at three<br />
different temperatures, 293.15, 323.15 <strong>and</strong> 353.15 K. Symbols correspond to the<br />
experimental data; dashed lines show calculations with soft-SAFT without the quadrupole,<br />
while the full lines represent calculations with the polar term. No binary parameters were<br />
fitted in these cases <strong>and</strong> so the lines are full predictions from the pure component<br />
parameters. It can be observed that the quadrupole has a significant influence on the<br />
description of the phase behaviour of the mixtures involving aromatic compounds. Polar<br />
soft-SAFT is able not only to provide an overall better agreement, but it also captures the<br />
changes in the curvature as a function of temperature. As before, the results given by the<br />
soft-SAFT EoS are compared with the ones given by the Peng-Robinson EoS represented<br />
in the figures by the lighter full lines. In the case of the CO2/aromatic compounds,<br />
especially for the CO2/perfluorobenzene mixture, the results given by the Peng-Robinson<br />
EoS are much better than could be expected when using such a simple equation.<br />
- 138 -