n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Experimental Methods, Results <strong>and</strong> Discussion<br />
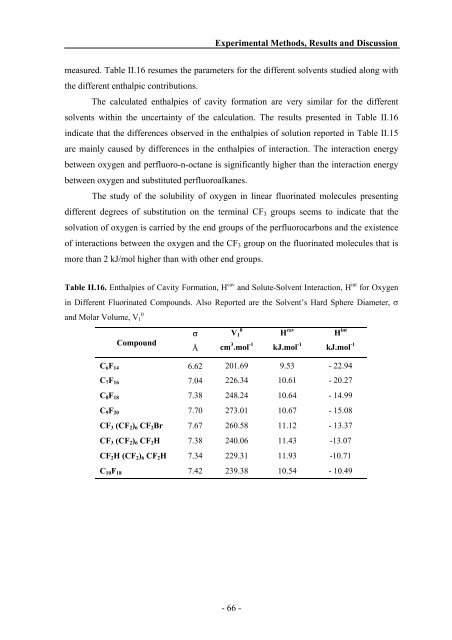
measured. Table II.16 resumes the parameters for the different solvents studied along with<br />
the different enthalpic contributions.<br />
The calculated enthalpies of cavity formation are very similar for the different<br />
solvents within the uncertainty of the calculation. The results presented in Table II.16<br />
indicate that the differences observed in the enthalpies of solution reported in Table II.15<br />
are mainly caused by differences in the enthalpies of interaction. The interaction energy<br />
between oxygen <strong>and</strong> perfluoro-n-octane is significantly higher than the interaction energy<br />
between oxygen <strong>and</strong> substituted perfluoroalkanes.<br />
The study of the solubility of oxygen in linear fluorinated molecules presenting<br />
different degrees of substitution on the terminal CF3 groups seems to indicate that the<br />
solvation of oxygen is carried by the end groups of the perfluorocarbons <strong>and</strong> the existence<br />
of interactions between the oxygen <strong>and</strong> the CF3 group on the fluorinated molecules that is<br />
more than 2 kJ/mol higher than with other end groups.<br />
Table II.16. Enthalpies of Cavity Formation, H cav <strong>and</strong> Solute-Solvent Interaction, H int for Oxygen<br />
in Different Fluorinated Compounds. Also Reported are the Solvent’s Hard Sphere Diameter, σ<br />
<strong>and</strong> Molar Volume, V1 0<br />
Compound<br />
σ<br />
Å<br />
V1 0<br />
cm 3 .mol -1<br />
H cav<br />
kJ.mol -1<br />
H int<br />
kJ.mol -1<br />
C6F14 6.62 201.69 9.53 - 22.94<br />
C7F16 7.04 226.34 10.61 - 20.27<br />
C8F18 7.38 248.24 10.64 - 14.99<br />
C9F20 7.70 273.01 10.67 - 15.08<br />
CF3 (CF2)6 CF2Br 7.67 260.58 11.12 - 13.37<br />
CF3 (CF2)6 CF2H 7.38 240.06 11.43 -13.07<br />
CF2H (CF2)6 CF2H 7.34 229.31 11.93 -10.71<br />
C10F18 7.42 239.38 10.54 - 10.49<br />
- 66 -