n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Experimental Methods, Results <strong>and</strong> Discussion<br />
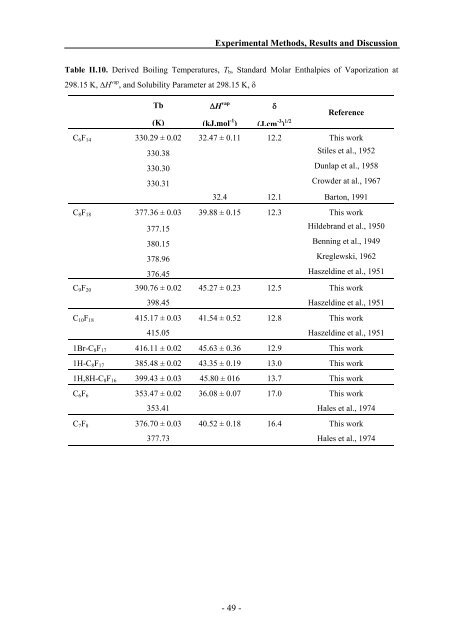
Table II.10. Derived Boiling Temperatures, Tb, St<strong>and</strong>ard Molar Enthalpies of Vaporization at<br />
298.15 K, ΔH vap , <strong>and</strong> Solubility Parameter at 298.15 K, δ<br />
Tb<br />
(K)<br />
ΔH vap<br />
(kJ.mol -1 )<br />
δ<br />
(J.cm -3 ) 1/2<br />
Reference<br />
C6F14 330.29 ± 0.02 32.47 ± 0.11 12.2 This work<br />
330.38 Stiles et al., 1952<br />
330.30 Dunlap et al., 1958<br />
330.31 Crowder at al., 1967<br />
32.4 12.1 Barton, 1991<br />
C8F18 377.36 ± 0.03 39.88 ± 0.15 12.3 This work<br />
377.15 Hildebr<strong>and</strong> et al., 1950<br />
380.15 Benning et al., 1949<br />
378.96 Kreglewski, 1962<br />
376.45 Haszeldine et al., 1951<br />
C9F20 390.76 ± 0.02 45.27 ± 0.23 12.5 This work<br />
398.45 Haszeldine et al., 1951<br />
C10F18 415.17 ± 0.03 41.54 ± 0.52 12.8 This work<br />
415.05 Haszeldine et al., 1951<br />
1Br-C8F17 416.11 ± 0.02 45.63 ± 0.36 12.9 This work<br />
1H-C8F17 385.48 ± 0.02 43.35 ± 0.19 13.0 This work<br />
1H,8H-C8F16 399.43 ± 0.03 45.80 ± 016 13.7 This work<br />
C6F6 353.47 ± 0.02 36.08 ± 0.07 17.0 This work<br />
353.41 Hales et al., 1974<br />
C7F8 376.70 ± 0.03 40.52 ± 0.18 16.4 This work<br />
377.73 Hales et al., 1974<br />
- 49 -