n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
General Introduction<br />
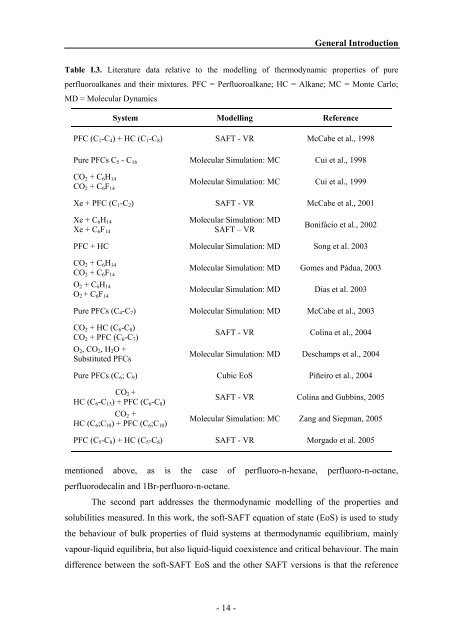
Table I.3. Literature data relative to the modelling of thermodynamic properties of pure<br />
perfluoroalkanes <strong>and</strong> their mixtures. PFC = Perfluoroalkane; HC = Alkane; MC = Monte Carlo;<br />
MD = Molecular Dynamics<br />
System Modelling Reference<br />
PFC (C1-C4) + HC (C1-C8) SAFT - VR McCabe et al., 1998<br />
Pure PFCs C5 - C16 Molecular Simulation: MC Cui et al., 1998<br />
CO2 + C6H14<br />
CO2 + C6F14<br />
Molecular Simulation: MC Cui et al., 1999<br />
Xe + PFC (C1-C2) SAFT - VR McCabe et al., 2001<br />
Xe + C6H14<br />
Xe + C6F14<br />
Molecular Simulation: MD<br />
SAFT – VR<br />
Bonifácio et al., 2002<br />
PFC + HC Molecular Simulation: MD Song et al. 2003<br />
CO2 + C6H14<br />
CO2 + C6F14<br />
O2 + C6H14<br />
O2 + C6F14<br />
Molecular Simulation: MD Gomes <strong>and</strong> Pádua, 2003<br />
Molecular Simulation: MD Dias et al. 2003<br />
Pure PFCs (C4-C7) Molecular Simulation: MD McCabe et al., 2003<br />
CO2 + HC (C6-C8)<br />
CO2 + PFC (C6-C7)<br />
O2, CO2, H2O +<br />
Substituted PFCs<br />
SAFT - VR Colina et al., 2004<br />
Molecular Simulation: MD Deschamps et al., 2004<br />
Pure PFCs (C6; C9) Cubic EoS Piñeiro et al., 2004<br />
CO2 +<br />
HC (C6-C13) + PFC (C6-C8)<br />
CO2 +<br />
HC (C6;C10) + PFC (C6;C10)<br />
SAFT - VR Colina <strong>and</strong> Gubbins, 2005<br />
Molecular Simulation: MC Zang <strong>and</strong> Siepman, 2005<br />
PFC (C5-C8) + HC (C5-C8) SAFT - VR Morgado et al. 2005<br />
mentioned above, as is the case of perfluoro-n-hexane, perfluoro-n-octane,<br />
perfluorodecalin <strong>and</strong> 1Br-perfluoro-n-octane.<br />
The second part addresses the thermodynamic modelling of the properties <strong>and</strong><br />
solubilities measured. In this work, the soft-SAFT equation of state (EoS) is used to study<br />
the behaviour of bulk properties of fluid systems at thermodynamic equilibrium, mainly<br />
vapour-liquid equilibria, but also liquid-liquid coexistence <strong>and</strong> critical behaviour. The main<br />
difference between the soft-SAFT EoS <strong>and</strong> the other SAFT versions is that the reference<br />
- 14 -