n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Experimental Methods, Results <strong>and</strong> Discussion<br />
distillation with more then 80 stages <strong>and</strong> fractional crystallization. Even so, Good et al.<br />
(1959) reported that after treatment with a neutral permanganate solution to remove<br />
olefinic impurities by oxidation followed by distillation in an efficient fractionating<br />
column <strong>and</strong> by silica gel percolation of selected fractions of distilled material, the purity of<br />
the best sample of perfluoromethylcyclohexane was only in the order of 98%.<br />
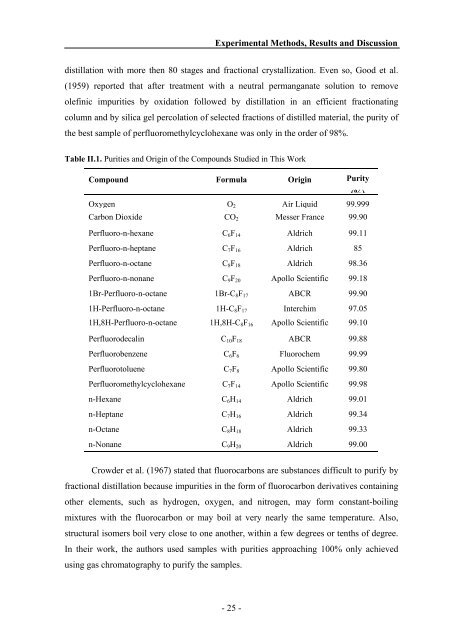
Table II.1. Purities <strong>and</strong> Origin of the Compounds Studied in This Work<br />
Compound Formula Origin Purity<br />
(%)<br />
Oxygen O2 Air Liquid 99.999<br />
Carbon Dioxide CO2 Messer France 99.90<br />
Perfluoro-n-hexane C6F14 Aldrich 99.11<br />
Perfluoro-n-heptane C7F16 Aldrich 85<br />
Perfluoro-n-octane C8F18 Aldrich 98.36<br />
Perfluoro-n-nonane C9F20 Apollo Scientific 99.18<br />
1Br-Perfluoro-n-octane 1Br-C8F17 ABCR 99.90<br />
1H-Perfluoro-n-octane 1H-C8F17 Interchim 97.05<br />
1H,8H-Perfluoro-n-octane 1H,8H-C8F16 Apollo Scientific 99.10<br />
Perfluorodecalin C10F18 ABCR 99.88<br />
Perfluorobenzene C6F6 Fluorochem 99.99<br />
Perfluorotoluene C7F8 Apollo Scientific 99.80<br />
Perfluoromethylcyclohexane C7F14 Apollo Scientific 99.98<br />
n-Hexane C6H14 Aldrich 99.01<br />
n-Heptane C7H16 Aldrich 99.34<br />
n-Octane C8H18 Aldrich 99.33<br />
n-Nonane C9H20 Aldrich 99.00<br />
Crowder et al. (1967) stated that fluorocarbons are substances difficult to purify by<br />
fractional distillation because impurities in the form of fluorocarbon derivatives containing<br />
other elements, such as hydrogen, oxygen, <strong>and</strong> nitrogen, may form constant-boiling<br />
mixtures with the fluorocarbon or may boil at very nearly the same temperature. Also,<br />
structural isomers boil very close to one another, within a few degrees or tenths of degree.<br />
In their work, the authors used samples with purities approaching 100% only achieved<br />
using gas chromatography to purify the samples.<br />
- 25 -