n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Modeling<br />
calculations from the original soft-SAFT equation <strong>and</strong> those provided from the polar soft-<br />
SAFT equation, being polar soft-SAFT superior than the simulations in this case. Note that<br />
in these simulations explicit charges were considered for the CO2 molecule.<br />
P / MPa<br />
14<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
0 0.2 0.4 0.6 0.8 1<br />
x, y CO2<br />
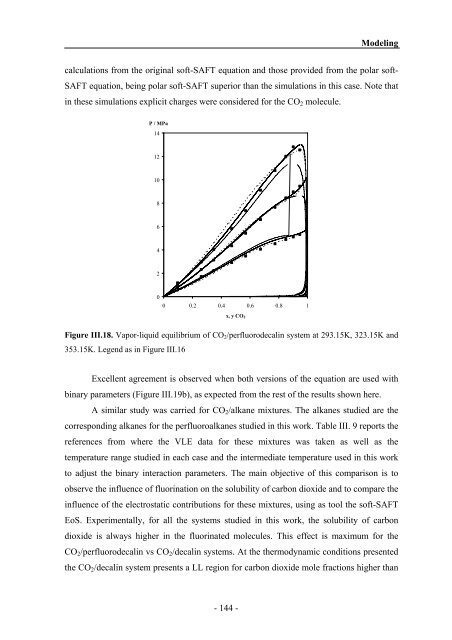
Figure III.18. Vapor-liquid equilibrium of CO2/perfluorodecalin system at 293.15K, 323.15K <strong>and</strong><br />
353.15K. Legend as in Figure III.16<br />
Excellent agreement is observed when both versions of the equation are used with<br />
binary parameters (Figure III.19b), as expected from the rest of the results shown here.<br />
A similar study was carried for CO2/alkane mixtures. The alkanes studied are the<br />
corresponding alkanes for the perfluoroalkanes studied in this work. Table III. 9 reports the<br />
references from where the VLE data for these mixtures was taken as well as the<br />
temperature range studied in each case <strong>and</strong> the intermediate temperature used in this work<br />
to adjust the binary interaction parameters. The main objective of this comparison is to<br />
observe the influence of fluorination on the solubility of carbon dioxide <strong>and</strong> to compare the<br />
influence of the electrostatic contributions for these mixtures, using as tool the soft-SAFT<br />
EoS. Experimentally, for all the systems studied in this work, the solubility of carbon<br />
dioxide is always higher in the fluorinated molecules. This effect is maximum for the<br />
CO2/perfluorodecalin vs CO2/decalin systems. At the thermodynamic conditions presented<br />
the CO2/decalin system presents a LL region for carbon dioxide mole fractions higher than<br />
- 144 -