n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Modeling<br />
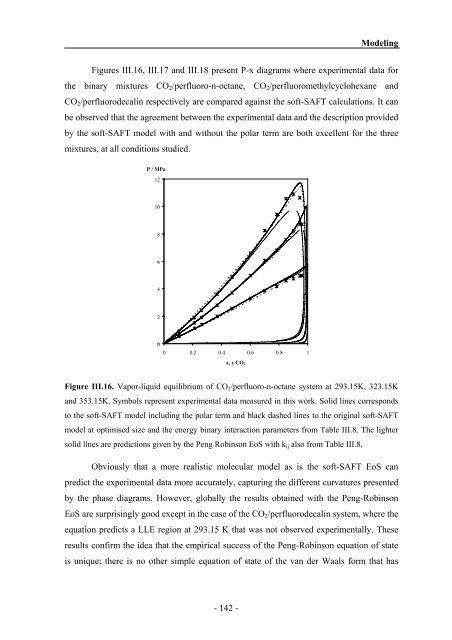
Figures III.16, III.17 <strong>and</strong> III.18 present P-x diagrams where experimental data for<br />
the binary mixtures CO2/perfluoro-n-octane, CO2/perfluoromethylcyclohexane <strong>and</strong><br />
CO2/perfluorodecalin respectively are compared against the soft-SAFT calculations. It can<br />
be observed that the agreement between the experimental data <strong>and</strong> the description provided<br />
by the soft-SAFT model with <strong>and</strong> without the polar term are both excellent for the three<br />
mixtures, at all conditions studied.<br />
P / MPa<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
0 0.2 0.4 0.6 0.8 1<br />
x, y CO2<br />
Figure III.16. Vapor-liquid equilibrium of CO2/perfluoro-n-octane system at 293.15K, 323.15K<br />
<strong>and</strong> 353.15K. Symbols represent experimental data measured in this work. Solid lines corresponds<br />
to the soft-SAFT model including the polar term <strong>and</strong> black dashed lines to the original soft-SAFT<br />
model at optimised size <strong>and</strong> the energy binary interaction parameters from Table III.8. The lighter<br />
solid lines are predictions given by the Peng Robinson EoS with kij also from Table III.8.<br />
Obviously that a more realistic molecular model as is the soft-SAFT EoS can<br />
predict the experimental data more accurately, capturing the different curvatures presented<br />
by the phase diagrams. However, globally the results obtained with the Peng-Robinson<br />
EoS are surprisingly good except in the case of the CO2/perfluorodecalin system, where the<br />
equation predicts a LLE region at 293.15 K that was not observed experimentally. These<br />
results confirm the idea that the empirical success of the Peng-Robinson equation of state<br />
is unique; there is no other simple equation of state of the van der Waals form that has<br />
- 142 -