n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
P / MPa<br />
14<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
0.0 0.2 0.4 0.6 0.8 1.0<br />
x, y CO2<br />
Modeling<br />
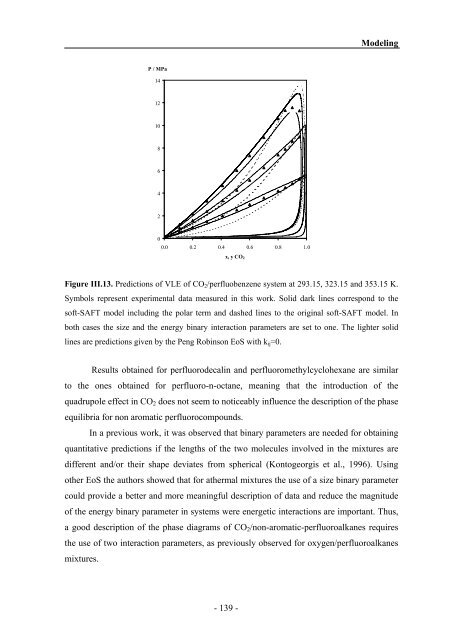
Figure III.13. Predictions of VLE of CO2/perfluobenzene system at 293.15, 323.15 <strong>and</strong> 353.15 K.<br />
Symbols represent experimental data measured in this work. Solid dark lines correspond to the<br />
soft-SAFT model including the polar term <strong>and</strong> dashed lines to the original soft-SAFT model. In<br />
both cases the size <strong>and</strong> the energy binary interaction parameters are set to one. The lighter solid<br />
lines are predictions given by the Peng Robinson EoS with kij=0.<br />
Results obtained for perfluorodecalin <strong>and</strong> perfluoromethylcyclohexane are similar<br />
to the ones obtained for perfluoro-n-octane, meaning that the introduction of the<br />
quadrupole effect in CO2 does not seem to noticeably influence the description of the phase<br />
equilibria for non aromatic perfluorocompounds.<br />
In a previous work, it was observed that binary parameters are needed for obtaining<br />
quantitative predictions if the lengths of the two molecules involved in the mixtures are<br />
different <strong>and</strong>/or their shape deviates from spherical (Kontogeorgis et al., 1996). Using<br />
other EoS the authors showed that for athermal mixtures the use of a size binary parameter<br />
could provide a better <strong>and</strong> more meaningful description of data <strong>and</strong> reduce the magnitude<br />
of the energy binary parameter in systems were energetic interactions are important. Thus,<br />
a good description of the phase diagrams of CO2/non-aromatic-perfluoroalkanes requires<br />
the use of two interaction parameters, as previously observed for oxygen/perfluoroalkanes<br />
mixtures.<br />
- 139 -