n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Experimental Methods, Results <strong>and</strong> Discussion<br />
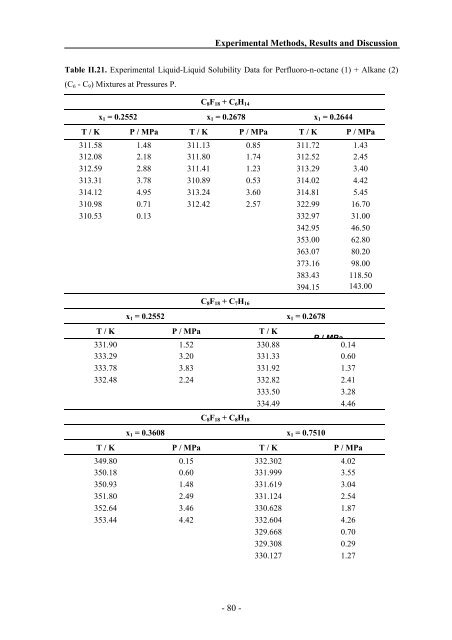
Table II.21. Experimental Liquid-Liquid Solubility Data for Perfluoro-n-octane (1) + Alkane (2)<br />
(C6 - C9) Mixtures at Pressures P.<br />
C8F18 + C6H14<br />
x1 = 0.2552 x1 = 0.2678 x1 = 0.2644<br />
T / K P / MPa T / K P / MPa T / K P / MPa<br />
311.58 1.48 311.13 0.85 311.72 1.43<br />
312.08 2.18 311.80 1.74 312.52 2.45<br />
312.59 2.88 311.41 1.23 313.29 3.40<br />
313.31 3.78 310.89 0.53 314.02 4.42<br />
314.12 4.95 313.24 3.60 314.81 5.45<br />
310.98 0.71 312.42 2.57 322.99 16.70<br />
310.53 0.13 332.97 31.00<br />
342.95 46.50<br />
353.00 62.80<br />
363.07 80.20<br />
373.16 98.00<br />
383.43 118.50<br />
394.15 143.00<br />
C8F18 + C7H16<br />
x1 = 0.2552 x1 = 0.2678<br />
T / K<br />
331.90<br />
P / MPa<br />
1.52<br />
T / K<br />
330.88<br />
P/MPa<br />
0.14<br />
333.29 3.20 331.33 0.60<br />
333.78 3.83 331.92 1.37<br />
332.48 2.24 332.82 2.41<br />
333.50 3.28<br />
334.49 4.46<br />
C8F18 + C8H18<br />
x1 = 0.3608 x1 = 0.7510<br />
T / K P / MPa T / K P / MPa<br />
349.80 0.15 332.302 4.02<br />
350.18 0.60 331.999 3.55<br />
350.93 1.48 331.619 3.04<br />
351.80 2.49 331.124 2.54<br />
352.64 3.46 330.628 1.87<br />
353.44 4.42 332.604 4.26<br />
329.668 0.70<br />
329.308 0.29<br />
330.127 1.27<br />
- 80 -