n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
n - PATh :.: Process and Product Applied Thermodynamics research ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
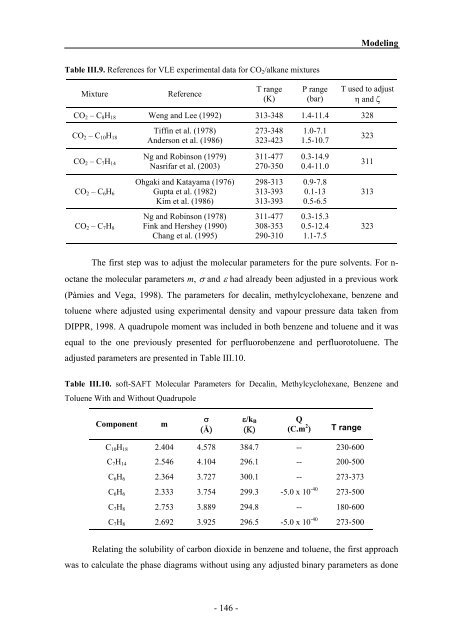
Table III.9. References for VLE experimental data for CO2/alkane mixtures<br />
Mixture Reference<br />
T range<br />
(K)<br />
P range<br />
(bar)<br />
Modeling<br />
T used to adjust<br />
η <strong>and</strong> ζ<br />
CO2 – C8H18 Weng <strong>and</strong> Lee (1992) 313-348 1.4-11.4 328<br />
CO2 – C10H18<br />
CO2 – C7H14<br />
CO2 – C6H6<br />
CO2 – C7H8<br />
Tiffin et al. (1978)<br />
Anderson et al. (1986)<br />
Ng <strong>and</strong> Robinson (1979)<br />
Nasrifar et al. (2003)<br />
Ohgaki <strong>and</strong> Katayama (1976)<br />
Gupta et al. (1982)<br />
Kim et al. (1986)<br />
273-348<br />
323-423<br />
311-477<br />
270-350<br />
298-313<br />
313-393<br />
313-393<br />
1.0-7.1<br />
1.5-10.7<br />
0.3-14.9<br />
0.4-11.0<br />
0.9-7.8<br />
0.1-13<br />
0.5-6.5<br />
Ng <strong>and</strong> Robinson (1978) 311-477 0.3-15.3<br />
Fink <strong>and</strong> Hershey (1990) 308-353 0.5-12.4<br />
Chang et al. (1995) 290-310 1.1-7.5<br />
The first step was to adjust the molecular parameters for the pure solvents. For n-<br />
octane the molecular parameters m, σ <strong>and</strong> ε had already been adjusted in a previous work<br />
(Pàmies <strong>and</strong> Vega, 1998). The parameters for decalin, methylcyclohexane, benzene <strong>and</strong><br />
toluene where adjusted using experimental density <strong>and</strong> vapour pressure data taken from<br />
DIPPR, 1998. A quadrupole moment was included in both benzene <strong>and</strong> toluene <strong>and</strong> it was<br />
equal to the one previously presented for perfluorobenzene <strong>and</strong> perfluorotoluene. The<br />
adjusted parameters are presented in Table III.10.<br />
Table III.10. soft-SAFT Molecular Parameters for Decalin, Methylcyclohexane, Benzene <strong>and</strong><br />
Toluene With <strong>and</strong> Without Quadrupole<br />
Component<br />
m<br />
σ<br />
(Å)<br />
ε/kBB<br />
(Κ)<br />
Q<br />
(C.m 2 )<br />
T range<br />
C10H18 2.404 4.578 384.7 -- 230-600<br />
C7H14 2.546 4.104 296.1 -- 200-500<br />
C6H6 2.364 3.727 300.1 -- 273-373<br />
C6H6 2.333 3.754 299.3 -5.0 x 10 -40<br />
273-500<br />
C7H8 2.753 3.889 294.8 -- 180-600<br />
-5.0 x 10 -40<br />
C7H8 2.692 3.925 296.5 273-500<br />
Relating the solubility of carbon dioxide in benzene <strong>and</strong> toluene, the first approach<br />
was to calculate the phase diagrams without using any adjusted binary parameters as done<br />
- 146 -<br />
323<br />
311<br />
313<br />
323