Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
4 Results and discussion<br />
of hydrolysis solution acidity on the microstructure of the Ti0.5Zr0.5O2 bulk material was<br />
studied with gas physisorption.<br />
The fractal dimension of the 50 mol% TiO2 <strong>in</strong> ZrO2 sol is larger (1.29) with similar<br />
particle size if H2O was used <strong>in</strong>stead of 1 M HNO3 as hydrolysis solution.<br />
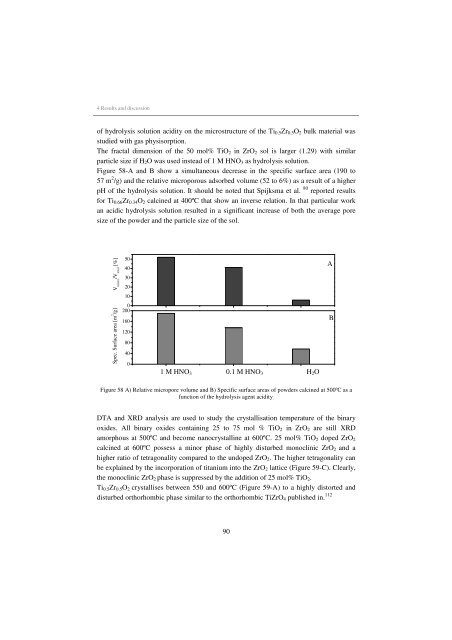
Figure 58-A and B show a simultaneous decrease <strong>in</strong> the specific surface area (190 to<br />
57 m 2 /g) and the relative microporous adsorbed volume (52 to 6%) as a result of a higher<br />
pH of the hydrolysis solution. It should be noted that Spijksma et al. 80 reported results<br />
<strong>for</strong> Ti0.66Zr0.34O2 calc<strong>in</strong>ed at 400ºC that show an <strong>in</strong>verse relation. In that particular work<br />
an acidic hydrolysis solution resulted <strong>in</strong> a significant <strong>in</strong>crease of both the average pore<br />
size of the powder and the particle size of the sol.<br />
V micro /V total [%]<br />
Spec. Surface area [m 2 /g]<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
200<br />
160<br />
120<br />
80<br />
40<br />
0<br />
1 M HNO3 0.1 M HNO3 H2O<br />
Figure 58 A) Relative micropore volume and B) Specific surface areas of powders calc<strong>in</strong>ed at 500ºC as a<br />
function of the hydrolysis agent acidity.<br />
DTA and XRD analysis are used to study the crystallisation temperature of the b<strong>in</strong>ary<br />
oxides. All b<strong>in</strong>ary oxides conta<strong>in</strong><strong>in</strong>g 25 to 75 mol % TiO2 <strong>in</strong> ZrO2 are still XRD<br />
amorphous at 500ºC and become nanocrystall<strong>in</strong>e at 600ºC. 25 mol% TiO2 doped ZrO2<br />
calc<strong>in</strong>ed at 600ºC possess a m<strong>in</strong>or phase of highly disturbed monocl<strong>in</strong>ic ZrO2 and a<br />
higher ratio of tetragonality compared to the undoped ZrO2. The higher tetragonality can<br />
be expla<strong>in</strong>ed by the <strong>in</strong>corporation of titanium <strong>in</strong>to the ZrO2 lattice (Figure 59-C). Clearly,<br />
the monocl<strong>in</strong>ic ZrO2 phase is suppressed by the addition of 25 mol% TiO2.<br />
Ti0.5Zr0.5O2 crystallises between 550 and 600ºC (Figure 59-A) to a highly distorted and<br />
disturbed orthorhombic phase similar to the orthorhombic TiZrO4 published <strong>in</strong>. 112<br />
90<br />
A<br />
B