Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
2 Theoretical background<br />
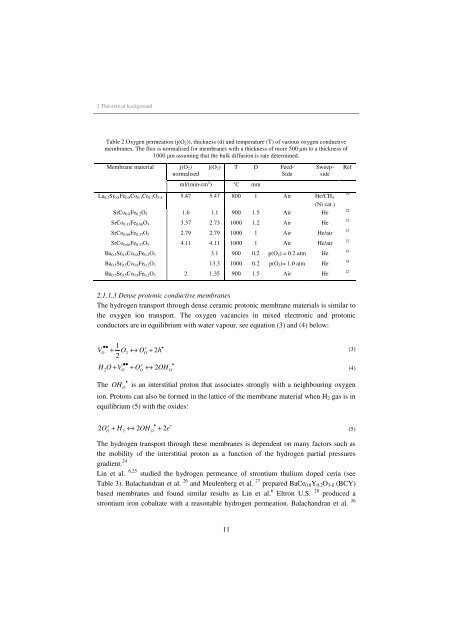
Table 2 Oxygen permeation (j(O2)), thickness (d) and temperature (T) of various oxygen conductive<br />
membranes. The flux is normalised <strong>for</strong> membranes with a thickness of more 500 µm to a thickness of<br />
1000 µm assum<strong>in</strong>g that the bulk diffusion is rate determ<strong>in</strong>ed.<br />
Membrane material<br />
j(O2)<br />
normalised<br />
j(O2) T D Feed-<br />
Side<br />
ml/(m<strong>in</strong>·cm 2 ) °C mm<br />
11<br />
Sweep-<br />
side<br />
La0.2Sr0.8Fe0.8Co0.1Cr0.1O3-δ 5.47 5.47 800 1 Air He/CH4<br />
(Ni cat.)<br />
SrCo0.8Fe0.2O3 1.6 1.1 900 1.5 Air He<br />
SrCo0.33Fe0.66O3 3.37 2.73 1000 1.2 Air He<br />
SrCo0.66Fe0.33O3 2.79 2.79 1000 1 Air He/air<br />
SrCo0.66Fe0.33O3 4.11 4.11 1000 1 Air He/air<br />
Ba0.5Sr0.5Co0.8Fe0.2O3 3.1 900 0.2 p(O2) = 0.2 atm He<br />
Ba0.5Sr0.5Co0.8Fe0.2O3 13.3 1000 0.2 p(O2)= 1.0 atm He<br />
Ba0.5Sr0.5Co0.8Fe0.2O3 2 1.35 900 1.5 Air He<br />
2.1.1.3 Dense protonic conductive membranes<br />
The hydrogen transport through dense ceramic protonic membrane materials is similar to<br />
the oxygen ion transport. The oxygen vacancies <strong>in</strong> mixed electronic and protonic<br />
conductors are <strong>in</strong> equilibrium with water vapour, see equation (3) and (4) below:<br />
•• 1<br />
x •<br />
VO + O2 ↔ OO + 2h<br />
2<br />
(3)<br />
x<br />
H O V O 2OH<br />
•<br />
••<br />
+ + ↔ (4)<br />
2<br />
O O O<br />
The OHO • is an <strong>in</strong>terstitial proton that associates strongly with a neighbour<strong>in</strong>g oxygen<br />
ion. Protons can also be <strong>for</strong>med <strong>in</strong> the lattice of the membrane material when H2 gas is <strong>in</strong><br />
equilibrium (5) with the oxides:<br />
2O + H ↔ 2OH + 2e′<br />
(5)<br />
x<br />
•<br />
O 2<br />
O<br />
The hydrogen transport through these membranes is dependent on many factors such as<br />
the mobility of the <strong>in</strong>terstitial proton as a function of the hydrogen partial pressures<br />
gradient. 24<br />
L<strong>in</strong> et al. 6,25 studied the hydrogen permeance of strontium thulium doped ceria (see<br />
Table 3). Balachandran et al. 26 and Meulenberg et al. 27 prepared BaCe0.8Y0.2O3-δ (BCY)<br />
based membranes and found similar results as L<strong>in</strong> et al. 6 Eltron U.S. 28 produced a<br />
strontium iron cobaltate with a reasonable hydrogen permeation. Balachandran et al. 26<br />
Ref<br />
19<br />
22<br />
23<br />
23<br />
23<br />
18<br />
18<br />
22