Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
4 Results and discussion<br />
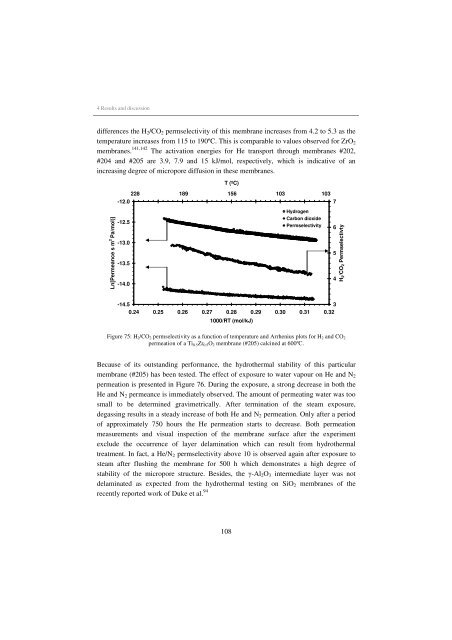
differences the H2/CO2 permselectivity of this membrane <strong>in</strong>creases from 4.2 to 5.3 as the<br />
temperature <strong>in</strong>creases from 115 to 190ºC. This is comparable to values observed <strong>for</strong> ZrO2<br />
membranes. 141,142 The activation energies <strong>for</strong> He transport through membranes #202,<br />
#204 and #205 are 3.9, 7.9 and 15 kJ/mol, respectively, which is <strong>in</strong>dicative of an<br />
<strong>in</strong>creas<strong>in</strong>g degree of micropore diffusion <strong>in</strong> these membranes.<br />
Ln[Permeance s m 2 Pa/mol)]<br />
0.24 228 0.25 0.26 189 0.27 0.28 156 0.29 0.30 103 0.31 103 0.32<br />
-12.0<br />
7<br />
-12.5<br />
-13.0<br />
-13.5<br />
-14.0<br />
T (ºC)<br />
-14.5<br />
3<br />
0.24 0.25 0.26 0.27 0.28 0.29 0.30 0.31 0.32<br />
1000/RT (mol/kJ)<br />
108<br />
Hydrogen<br />
Carbon dioxide<br />
Permselectivity<br />
Figure 75: H2/CO2 permselectivity as a function of temperature and Arrhenius plots <strong>for</strong> H2 and CO2<br />
permeation of a Ti0.5Zr0.5O2 membrane (#205) calc<strong>in</strong>ed at 600ºC.<br />
Because of its outstand<strong>in</strong>g per<strong>for</strong>mance, the hydrothermal stability of this particular<br />
membrane (#205) has been tested. The effect of exposure to water vapour on He and N2<br />
permeation is presented <strong>in</strong> Figure 76. Dur<strong>in</strong>g the exposure, a strong decrease <strong>in</strong> both the<br />
He and N2 permeance is immediately observed. The amount of permeat<strong>in</strong>g water was too<br />
small to be determ<strong>in</strong>ed gravimetrically. After term<strong>in</strong>ation of the steam exposure,<br />
degass<strong>in</strong>g results <strong>in</strong> a steady <strong>in</strong>crease of both He and N2 permeation. Only after a period<br />
of approximately 750 hours the He permeation starts to decrease. Both permeation<br />
measurements and visual <strong>in</strong>spection of the membrane surface after the experiment<br />
exclude the occurrence of layer delam<strong>in</strong>ation which can result from hydrothermal<br />
treatment. In fact, a He/N2 permselectivity above 10 is observed aga<strong>in</strong> after exposure to<br />
steam after flush<strong>in</strong>g the membrane <strong>for</strong> 500 h which demonstrates a high degree of<br />
stability of the micropore structure. Besides, the γ-Al2O3 <strong>in</strong>termediate layer was not<br />
delam<strong>in</strong>ated as expected from the hydrothermal test<strong>in</strong>g on SiO2 membranes of the<br />
recently reported work of Duke et al. 94<br />
6<br />
5<br />
4<br />
H 2/CO 2 Permselectivty