Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
Inorganic Microporous Membranes for Gas Separation in Fossil Fuel ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
4 Results and discussion<br />
Permeance (10 -9 mol/s m2Pa)<br />
Permeance (10 -9 mol/s m 2 Pa)<br />
1.E-06<br />
10 -6<br />
1.E-07<br />
10 -7<br />
1.E-08<br />
10 -8<br />
1.E-09<br />
10 -9<br />
1.E-10 1E-08<br />
10 -10<br />
10 -8<br />
10 -9<br />
1E-09<br />
10 -10<br />
107<br />
#204; 600ºC<br />
#205; 600ºC<br />
1E-10<br />
0.2 0.3 0.4 0.5 0.6 0.7 0.8<br />
K<strong>in</strong>etic diameter (nm)<br />
#143; 500ºC<br />
#132; 500ºC<br />
#202; 500ºC<br />
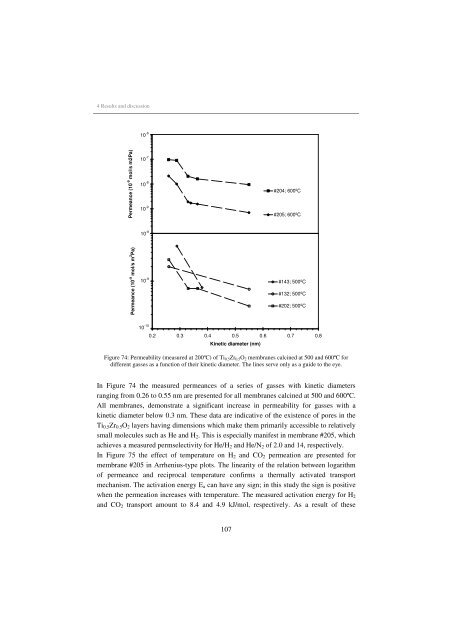
Figure 74: Permeability (measured at 200ºC) of Ti0.5Zr0.5O2 membranes calc<strong>in</strong>ed at 500 and 600ºC <strong>for</strong><br />
different gasses as a function of their k<strong>in</strong>etic diameter. The l<strong>in</strong>es serve only as a guide to the eye.<br />
In Figure 74 the measured permeances of a series of gasses with k<strong>in</strong>etic diameters<br />
rang<strong>in</strong>g from 0.26 to 0.55 nm are presented <strong>for</strong> all membranes calc<strong>in</strong>ed at 500 and 600ºC.<br />
All membranes, demonstrate a significant <strong>in</strong>crease <strong>in</strong> permeability <strong>for</strong> gasses with a<br />
k<strong>in</strong>etic diameter below 0.3 nm. These data are <strong>in</strong>dicative of the existence of pores <strong>in</strong> the<br />
Ti0.5Zr0.5O2 layers hav<strong>in</strong>g dimensions which make them primarily accessible to relatively<br />
small molecules such as He and H2. This is especially manifest <strong>in</strong> membrane #205, which<br />
achieves a measured permselectivity <strong>for</strong> He/H2 and He/N2 of 2.0 and 14, respectively.<br />
In Figure 75 the effect of temperature on H2 and CO2 permeation are presented <strong>for</strong><br />
membrane #205 <strong>in</strong> Arrhenius-type plots. The l<strong>in</strong>earity of the relation between logarithm<br />
of permeance and reciprocal temperature confirms a thermally activated transport<br />
mechanism. The activation energy Ea can have any sign; <strong>in</strong> this study the sign is positive<br />
when the permeation <strong>in</strong>creases with temperature. The measured activation energy <strong>for</strong> H2<br />
and CO2 transport amount to 8.4 and 4.9 kJ/mol, respectively. As a result of these